Figure 2.
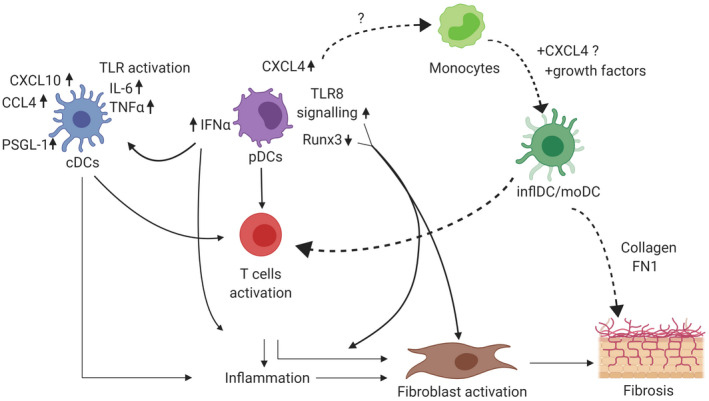
Plasmacytoid dendritic cells (pDCs) and conventional dendritic cells (cDCs) have a crucial role in the inflammatory and fibrotic processes in systemic sclerosis (SSc). pDCs produce a large amount of type I interferon (IFN)‐α and chemokine C‐X‐C motif ligand 4 (CXCL4) due to dysregulated mechanisms such as runt‐related transcription factor 3 (RUNX3) and Toll‐like receptor (TLR)‐8 signalling. IFN‐α has a strong capacity to induce inflammation and to activate other innate immune cells such as cDCs. Exacerbated TLR activation in SSc cDCs lead to an increased production of cytokines and chemokines as, for example, CXCL10, chemokine (C‐C motif) ligand 4 (CCL4), IL‐6, TNF‐α and cell adhesion molecules such as P‐selectin glycoprotein ligand 1 (PSGL‐1). Activated cDCs display a higher ability to induce T cell activation. Additionally, exacerbated CXCL4 production by pDCs might modulate monocytes differentiation into monocyte‐derived inflammatory DCs (inflDC) with an enhanced cytokine production capacity upon TLR (Toll‐like receptor) stimulation, a superior T cell stimulation and a profibrotic phenotype. As a result of these dysregulated mechanisms, DCs promote inflammation, myofibroblast transformation and extracellular matrix (ECM) deposition in the affected tissue of SSc patients.
