Summary
Women who conceive at 35 years of age or older, commonly known as advanced maternal age, have a higher risk of facing parturition complications and their children have an increased risk of developing diseases later in life. However, the immunological mechanisms underlying these pathological processes have yet to be established. To fill this gap in knowledge, using a murine model and immunophenotyping, we determined the effect of advanced maternal age on the main cellular branch of adaptive immunity, T cells, at the maternal–fetal interface and in the offspring. We report that advanced maternal age impaired the process of labor at term, inducing dystocia and delaying the timing of delivery. Advanced maternal age diminished the number of specific proinflammatory T‐cell subsets [T helper type 1 (Th1): CD4+IFN‐γ+, CD8+IFN‐γ+ and Th9: CD4+IL‐9+], as well as CD4+ regulatory T cells (CD4+CD25+FoxP3+ T cells), at the maternal–fetal interface prior to term labor. Advanced maternal age also altered fetal growth and survival of the offspring in early life. In addition, infants born to advanced‐age mothers had alterations in the T‐cell repertoire but not in CD71+ erythroid cells (CD3−CD71+TER119+ cells). This study provides insight into the immune alterations observed at the maternal–fetal interface of advanced‐age mothers and their offspring.
Keywords: birth weight, neonate, offspring, pregnancy, preterm labor
Advanced maternal age alters the T‐cell repertoire at the maternal–fetal interface prior to term labor. Importantly, infants born to advance aged dams also display an impaired T‐cell repertoire.

Introduction
During the past three decades, the mean childbearing age has steadily increased in developed and high‐income countries, due largely to social and career‐based changes as well as advances in contraceptives and assisted reproductive technologies [1]. Women aged 35 years or older, commonly defined as being of advanced maternal age, now comprise a significant proportion of the pregnant population [1, 2]. Such delayed pregnancy is associated with a wide range of perinatal complications, including a higher risk of developing hypertensive disorders [3, 4, 5] and gestational diabetes mellitus [6, 7]. Additionally, women of advanced maternal age more commonly face parturition complications such as dystocia/prolonged labor [8, 9], indicated cesarean section [10, 11, 12] and maternal near‐miss events or morbidity [13, 14]. While the associations between advanced maternal age and these pregnancy complications are well established, the immunological mechanisms underlying these pathological processes, particularly prolonged labor, are poorly understood.
Labor is considered a state of systemic [15, 16, 17, 18, 19] and local [20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31] physiological inflammation [32, 33, 34, 35]. The latter concept is supported by consistent evidence showing that labor is characterized by an increase in cellular and soluble inflammatory mediators in the cervix [32, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48], myometrium [37, 38, 40, 49, 50, 51, 52], chorioamniotic membranes [38, 40, 53, 54, 55, 56, 57, 58, 59], and decidual tissues (i.e. maternal–fetal interface) [38, 40, 53, 54, 57, 60, 61, 62, 63]. Specifically, in the decidual tissues, the process of labor at term has been associated with proinflammatory phenotypes of macrophages (i.e. M1‐like phenotype) [64] and effector T cells [65, 66]. Indeed, these immune cell types are also detected in women who underwent the pathological process of preterm labor [64, 65]. Therefore, we have proposed that a tight balance among the cellular components at the maternal–fetal interface is implicated in the physiological and pathological processes of labor [67, 68, 69]. However, whether alterations in the adaptive immune responses, specifically T cells, take place at the maternal–fetal interface in women of advanced maternal age has yet to be shown.
Beyond the pregnancy consequences associated with advanced maternal age, several studies reported that the children born to women of advanced maternal age have an increased risk of developing diseases later in life, including type 1 childhood diabetes [70, 71, 72], allergies [73], male infertility [74] and female menstrual disorders [75], among others [76, 77, 78, 79, 80, 81, 82]. While the etiology of some of these long‐term sequelae remains unknown, most have no known linkage to maternal transmission of genes, defective mitochondria, or chromosomal abnormalities [83]. Furthermore, there is an increasing body of evidence suggesting that the intrauterine environment shapes developmental outcomes, including immunological development in the offspring [84, 85, 86]. This hypothesis, in tandem with the understanding that aging is characterized by chronic systemic inflammation [87] and that pregnancy is tightly regulated by the immune system [88, 89, 90, 91], begs the question of how the intrauterine environment in women of advanced maternal age may alter T‐cell responses in the offspring.
In the current study, we first evaluated the perinatal consequences of advanced maternal age using a murine model. Additionally, we performed immunophenotyping of decidual and splenic infantile murine T cells to determine the impact of advanced maternal age on the main cellular branch of adaptive immunity at the maternal–fetal interface and in the offspring, respectively.
Materials and methods
Mice
B6N.129(Cg)‐forkhead box protein 3 (FoxP3)tm3Ayr/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), bred in the animal care facility at the C. S. Mott Center for Human Growth and Development (Wayne State University, Detroit, MI, USA), and housed under a circadian cycle (light/dark = 12 : 12 h). This mouse strain was chosen because initial studies were focused on regulatory T cells (Tregs). Syngeneic mating was used to evaluate the effect of advanced maternal age as the sole variable. Older females [≥ 20 weeks, advanced maternal age (AMA)] were mated with males of proven fertility in three different cohorts: the first to obtain observational data and the second two for flow cytometry data. Young females (aged 8–12 weeks, ideal reproductive age, controls) were also mated with males of proven fertility as controls. The females were checked between 8:00 a.m. and 9:00 a.m. daily for the appearance of a vaginal plug indicating 0·5 days post‐coitum (dpc), at which point female mice were removed from the mating cages and housed separately. Pregnancy was confirmed by a weight gain of ≥ 2 g at 12·5 dpc. All mouse experiments were approved by the Institutional Animal Care and Use Committee at Wayne State University (Protocol no. A‐09‐08‐12). The authors adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Murine model of advanced maternal age
In the first cohort, fertility rates were recorded, defined as the proportion of mice who successfully became pregnant after the identification of a vaginal plug among the total number of mice with a vaginal plug. Pregnancy parameters, including duration of labor, rate of dystocia, and gestational length, were monitored via video camera (Sony, Tokyo, Japan). Duration of labor was defined as the time between delivery of the first and last pup in undisturbed, successful deliveries. The rate of dystocia was defined as the proportion of mice who underwent disturbed progression of labor (duration of labor > 6 h) among the total number of pregnant mice. Gestational length was calculated as the time from the presence of the vaginal plug until the observation of the first pup in the cage bedding. Litter sizes of all successful deliveries were recorded. After delivery, the mother and her pups were kept under observation, and offspring survival and weights were recorded 1, 2, and 3 weeks after birth.
Immunophenotyping by flow cytometry
Older and young dams from the second cohort were euthanized prior to term parturition, the day before delivery, on 18·5 dpc. The number of fetuses, fetal weights, and placental weights were recorded. Additionally, the maternal spleen, uterine draining lymph nodes (ULN), and decidual tissues were collected. In the third cohort, neonates (1 week) and infants (3 weeks) from older and young dams were weighed and euthanized, and spleens were collected. The isolation of leukocytes from lymphatic and decidual tissues was performed using mechanical dissociation followed by enzymatic disaggregation, as previously described [67, 92]. The cells were incubated with anti‐CD16/CD32 (FcγIII/II receptor; BD Biosciences, San Jose, CA, USA) for 10 min, followed by extracellular staining with specific fluorochrome‐conjugated anti‐mouse monoclonal antibodies (Supporting information, Table S1). For intracellular/intranuclear staining, the cells were first fixed and permeabilized using the Cytofix/Cytoperm fixation/permeabilization solution (Cat. no. 554714; BD Biosciences) or the FoxP3 staining buffer kit (Cat. no. 005523‐00; eBiosciences, San Diego, CA, USA), respectively, prior to incubation with intracellular/intranuclear antibodies, which included staining for cytokines (Supporting information, Table S1). For the staining of CD71+ erythroid cells, the 1X fluorescence activated cell sorter (FACS) lysing solution (BD Biosciences) was used. After staining, cell pellets were washed and resuspended in 0·5 ml FACS buffer. Samples were acquired using the BD LSRFortessa® flow cytometer (BD Biosciences) and analyzed with BD FACSDiva® Software version 7.0 (BD Biosciences). The analysis and figures were performed using FlowJo software version 10 (FlowJo, LLC, Ashland, OR, USA). The absolute number of cells was determined using CountBright absolute counting beads (Molecular Probes, Eugene, OR, USA).
T‐cell phenotypes were determined in the maternal tissues and infantile tissues. Such immunophenotyping included the identification of: conventional T cells (CD3+), CD4+ T cells (CD3+CD4+), CD8+ T cells (CD3+CD8+), T helper type 1 (Th1) cells [CD3+CD4+IFN‐γ+], Th2 cells [CD3+CD4+IL‐4+], Th9 cells (CD3+CD4+IL‐9+), Th17 cells (CD3+CD4+IL‐17A+), CD8+IFN‐γ+ cells (CD3+CD8+IFN‐γ+), CD8+IL‐4+ cells (CD3+CD8+IL‐4+), CD8+IL‐9+ cells (CD3+CD8+IL‐9+), CD8+IL‐17A+ cells (CD3+CD8+IL‐17A+), CD4+ regulatory T cells (CD3+CD4+CD25+FoxP3+) and CD3+CD8+CD25+FoxP3+ cells. CD71+ erythroid cells (CD3−CD71+TER119+) were also identified in neonatal and infantile tissues and reported as proportions due to low cell counts.
Statistical analysis
Data were analyzed using SPSS Statistics software version 19.0 (IBM, Armonk, NY, USA). For the rates of fertility and dystocia, Fisher’s exact test was used. Kaplan–Meier survival curves were used to plot and compare the gestational length and neonatal survival data (Mantel–Cox test). For the duration of labor, litter size, placental weights offspring weights, and all flow cytometry data, the Shapiro–Wilk normality test was performed. For non‐normally distributed data, the Mann–Whitney U‐test was utilized to compare experimental data between the control and study groups. Alternatively, for normally distributed data, the unpaired t‐test was performed. A P‐value of ≤ 0·05 was considered statistically significant.
Results
The negative effects of advanced maternal age in pregnancy outcomes
Mice reach sexual maturity at ~6–8 weeks of age [93]; however, the mating age used in reproductive studies is between 8 and 12 weeks of age [65, 94, 95]. Therefore, in the current study, control mice were mated within this range (8–12 weeks of age), which represents the ideal reproductive age (Fig. 1a). In rodents, fertility begins to decline at approximately 6 months of age (~24 weeks), which mirrors the decline in fertility of women who are aged ~35–40 years [96]. Therefore, older females were allowed to reach 20–24 weeks of age, which represents the decline in fertility seen in women of advanced maternal age (AMA, Fig. 1a). Consistently, we found that AMA dams tended to have a lower fertility rate compared to controls [73.9% (17 of 23) versus 100% (13 of 13), Fig. 1b]. In addition, AMA dams that successfully reached term pregnancy had significantly longer durations of labor than controls (Fig. 1c), which is consistent with human data associating prolonged labor with increased maternal age [9]. Furthermore, the rate of dystocia was notably higher in the AMA group than the control group [35.2% (six of 17) versus 7·7% (one of 13), Fig. 1d]. The gestational length of AMA dams tended to be longer than that of controls (Fig. 1e). However, the litter size between AMA and control dams was not significantly different (Fig. 1f). These data show that advanced maternal age is associated with adverse effects during pregnancy, including impaired fertility, increased duration of labor and gestation and a higher frequency of dystocia.
Fig. 1.
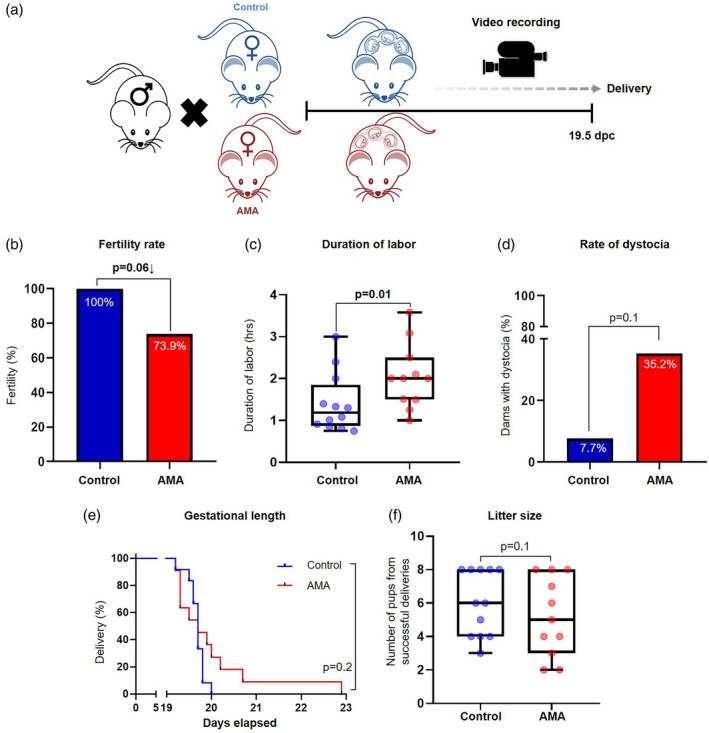
Pregnancy outcomes of advanced maternal age. (a) Experimental design of advanced maternal age (AMA) during pregnancy. (b) Percentage of fertile control and AMA mice (n = 13–23 each). The P‐values were determined by Fisher’s exact test. (c) Duration of active labor in undisturbed, successful deliveries from control and AMA dams (n = 11–12 each) in control and AMA groups. Mid‐lines indicate medians, boxes indicate interquartile ranges and whiskers indicate minimum–maximum range. The P‐values were determined by a Mann–Whitney U‐test. (d) Percentage of control and AMA dams who went into dystocia (n = 13–17 each). The P‐values were determined by Fisher’s exact test. (e) Kaplan–Meier survival curves showing the gestational lengths of control and AMA dams (n = 11–12 each). The P‐values were determined by Mantel–Cox test. (f) Number of pups per litter from control and AMA dams who had successful deliveries (n = 11–12 litters). Mid‐lines indicate medians, boxes indicate interquartile ranges and whiskers indicate minimum–maximum range. The P‐values were determined by a Mann–Whitney U‐test. Significant P‐values are shown in bold type.
Advanced maternal age alters the T‐cell repertoire at the maternal–fetal interface
Previous studies have shown that T cells at the maternal–fetal interface participate in the physiological [57, 65, 66, 69, 97, 98, 99] and pathological [65, 69, 99, 100, 101, 102, 103] processes of labor. Therefore, we next quantified decidual T‐cell subsets prior to term delivery (18·5 dpc) using flow cytometry (Fig. 2a,b). We first determined the numbers of conventional T cells in the decidua, and found no differences in the decidual CD4+ (Fig. 2c) or CD8+ (Fig. 2d) T cells between AMA dams and controls. We next analyzed the expression of stereotypical cytokines associated with helper T‐cell subsets: namely, IFN‐γ (Th1) [104, 105], IL‐4 (Th2) [104, 105], IL‐9 (Th9) [106, 107, 108, 109], and IL‐17A (Th17) [110] (Fig. 2b, right panels). Advanced maternal age was associated with a marked reduction in decidual Th1 cells compared to controls (Fig. 2e). Moreover, a modest but non‐significant reduction was observed in the numbers of decidual Th9 cells in AMA dams compared to controls (Fig. 2g; P = 0·05). Neither the number of decidual Th2 cells (Fig. 2f) nor the number of Th17 cells (Fig. 2h) were significantly altered by AMA. Among the corresponding CD8+ T‐cell subsets, there was a significant reduction in the population of CD8+IFN‐γ+ T cells in AMA dams compared to controls (Fig. 2i), similar to the trend seen in the Th1 cells. There was also a modest decrease in CD8+IL‐4+ T cells in AMA dams compared to controls (Fig. 2j; P = 0·06). No differences were seen in the number of CD8+IL‐9+ T cells (Fig. 2k) or CD8+IL‐17A+ T cells (Fig. 2l) between the study groups. Notably, such alterations in T‐cell populations were limited to the maternal–fetal interface, as no changes were observed in T‐cell subsets in the maternal ULN or spleen between AMA dams and controls (Supporting information, Figs [Link], [Link]). Together, these results show that AMA impairs the proinflammatory T‐cell responses at the maternal–fetal interface prior to parturition.
Fig. 2.
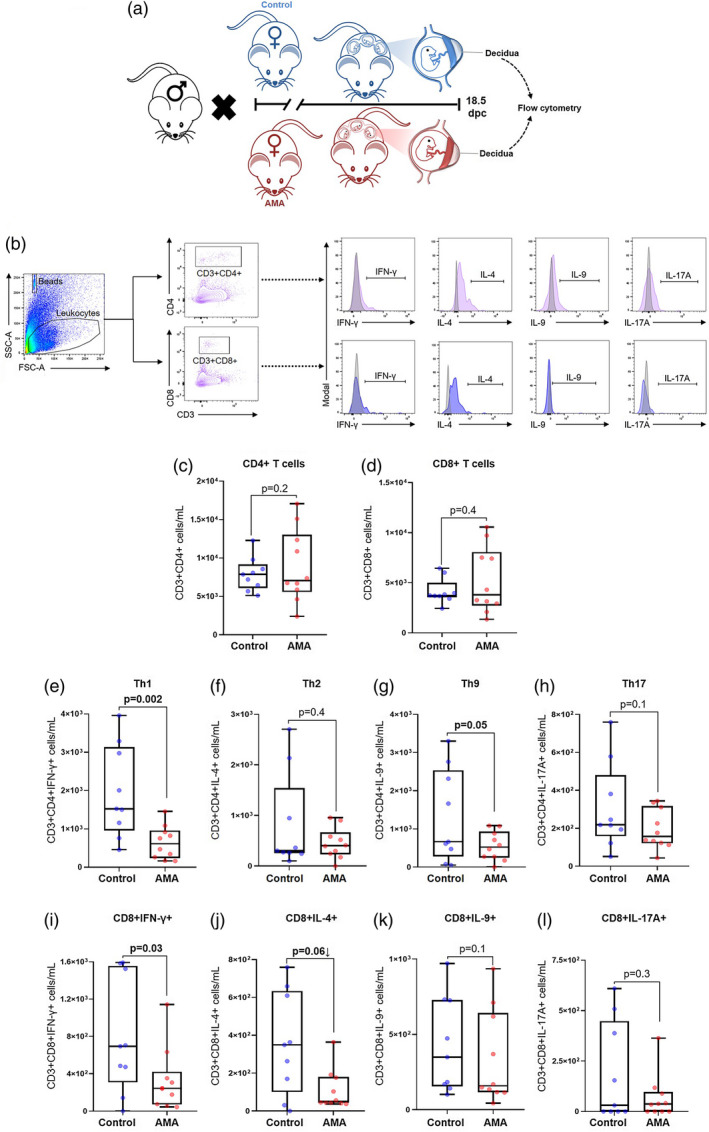
Immunophenotyping of the T‐cell subsets in the decidua of advanced maternal aged dams. (a) Experimental design of decidual tissue collection in murine model of advanced maternal age (AMA) and young controls. (b) Gating strategy used to quantify the T‐cell subsets in the decidua. Number of (c) CD4+ T cells, (d) CD8+ T cells, (e) T helper type 1 (Th1) cells, (f) Th2 cells, (g) Th9 cells, (h) Th17 cells, (i) CD8+ cells expressing IFN‐γ, (j) CD8+ cells expressing IL‐4, (k) CD8+ cells expressing IL‐9, and (l) CD8+ cells expressing IL‐17A (n = 9–10 each). Mid‐lines indicate medians, boxes indicate interquartile ranges and whiskers indicate minimum–maximum range. The P‐values were determined by an unpaired t‐test or a Mann–Whitney U‐test. Significant P‐values are shown in bold type.
We and others have previously suggested that systemic and local Tregs play an important role in the timing of parturition [98, 111, 112, 113, 114, 115]. Therefore, we quantified CD4+ Tregs and CD8+CD25+FoxP3+ T cells in the decidual tissues of AMA and control dams (Fig. 3a). We found that AMA dams had a significantly diminished population of decidual CD4+ Tregs compared to controls (Fig. 3b). However, this reduction in CD4+ Tregs was exclusively local, as no changes were observed in Tregs from the ULN or spleen of AMA and control dams (Supporting information, Fig. S3). By contrast, no differences were seen in the numbers of CD8+CD25+FoxP3+ T cells between study groups (Fig. 3c). This finding demonstrates that AMA results in a reduction of CD4+ Tregs at the maternal–fetal interface, but does not affect the CD8+CD25+FoxP3+ T‐cell population.
Fig. 3.
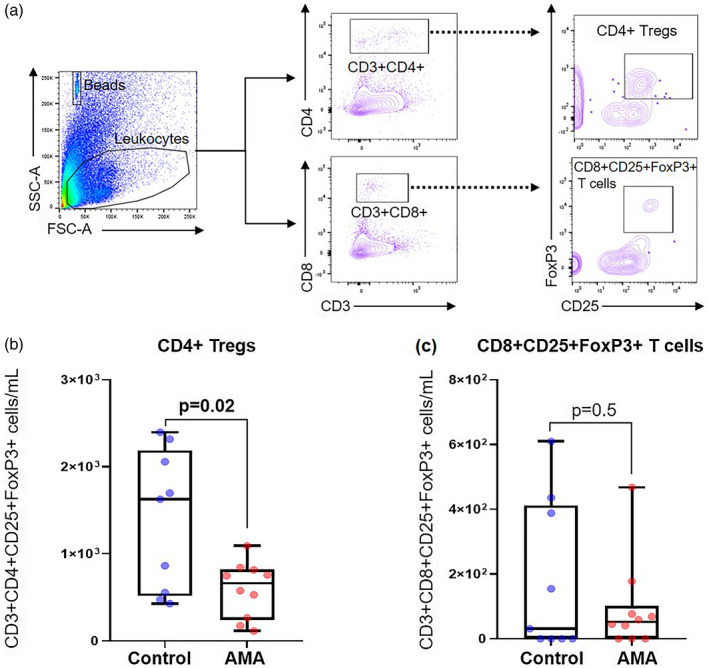
Immunophenotyping of regulatory T cells in the decidua of advanced maternal aged (AMA) dams. (a) Gating strategy used to quantify the regulatory T cells (Tregs) in the decidua. Number of (b) CD4+ Tregs and (c) CD8+CD25+FoxP3+ T cells (n = 9–10 each) in control and AMA dams. Mid‐lines indicate medians, boxes indicate interquartile ranges and whiskers indicate minimum–maximum range. The P‐values were determined by an unpaired t‐test or a Mann–Whitney U‐test. Significant P‐values are shown in bold type.
Advanced maternal age impairs neonatal survival and growth of the offspring
Given that AMA has been associated with adverse neonatal outcomes such as low birth weight [116] and a higher rate of stillbirth [117, 118], we next investigated the impact of older maternal age on the immediate and long‐term health of the offspring (Fig. 4a). There was a significant reduction in neonatal survival in the AMA group compared to controls, with neonatal mortality predominately occurring within the first week of life among the different litters (Fig. 4b). To further elucidate this discrepancy in neonatal survival and assess the long‐term health of the offspring, placental and fetal weights at 18·5 dpc as well as neonatal growth trajectories were compared between AMA mice and controls. There was no difference in the placental weights at 18·5 dpc between the study groups (Fig. 4c). However, there was a significant reduction in the fetal weights from AMA dams at 18·5 dpc compared to controls (Fig. 4d), indicating that the pup‐to‐placenta weight ratio contributes to AMA‐related neonatal consequences. Interestingly, neonates born to AMA dams were significantly heavier than those from young controls at 1 week of age (Fig. 4e). This disparity in offspring growth was overcome by 3 weeks of age, as a difference was not observed between the infants from AMA dams and controls (Fig. 4f). These results provide supporting evidence showing that AMA not only impairs the process of labor, but also affects the early survival and growth of the offspring.
Fig. 4.
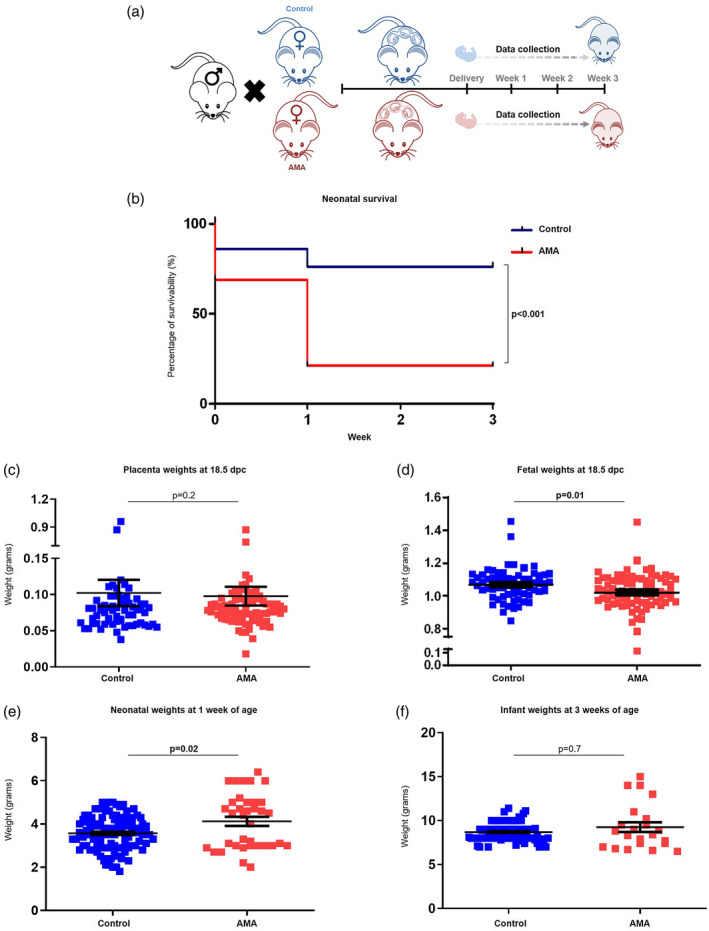
Neonatal outcomes in advanced maternal aged dams. (a) Experimental design of data collection from the offspring of advanced maternal aged (AMA) and control dams. (b) Kaplan–Meier survival curves showing the rate of survival of offspring from AMA dams and controls at birth and 1, 2, and 3 weeks of life. The P‐values were determined by Mantel–Cox test. (c) Weights of the placentas from controls and AMA dams at 18·5 days post‐coitum (dpc). Weights of the offspring from control and AMA dams at (d) 18·5 dpc, (e) 1 week and (f) 3 weeks of age (n = 22–103 each). Data are shown as scatter‐plots mean with standard error of the mean (s.e.m.)]. The P‐values were determined by a Mann–Whitney U‐test. Significant P‐values are shown in bold type.
Advanced maternal age disrupts T‐cell phenotypes in infants
Infants born to AMA dams were apparently healthy; however, there is a substantial body of literature associating advanced maternal age with multiple disorders in the offspring later in life [70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83]. Therefore, we evaluated T‐cell responses in infants born to AMA dams to determine the integrity of the cellular limb of their adaptive immune system. First, we characterized the conventional T‐cell repertoire in the infant spleen (Fig. 5a,b). Infants from AMA dams had modestly increased numbers of splenic CD4+ T cells compared to those from controls (Fig. 5c; P = 0·05). Moreover, the numbers of splenic CD8+ T cells were also elevated in infants from AMA dams compared to those from controls (Fig. 5d). We next investigated splenic helper T‐cell subsets in the offspring of AMA dams, and found that the numbers of Th1 cells were significantly increased compared to the offspring of controls (Fig. 5e). Similarly, splenic Th2 T cells were also augmented in infants from AMA dams (Fig. 5f; P = 0·05). However, the numbers of Th9 cells (Fig. 5g) and Th17 cells (Fig. 5h) remained stable when compared between the study groups.
Fig. 5.
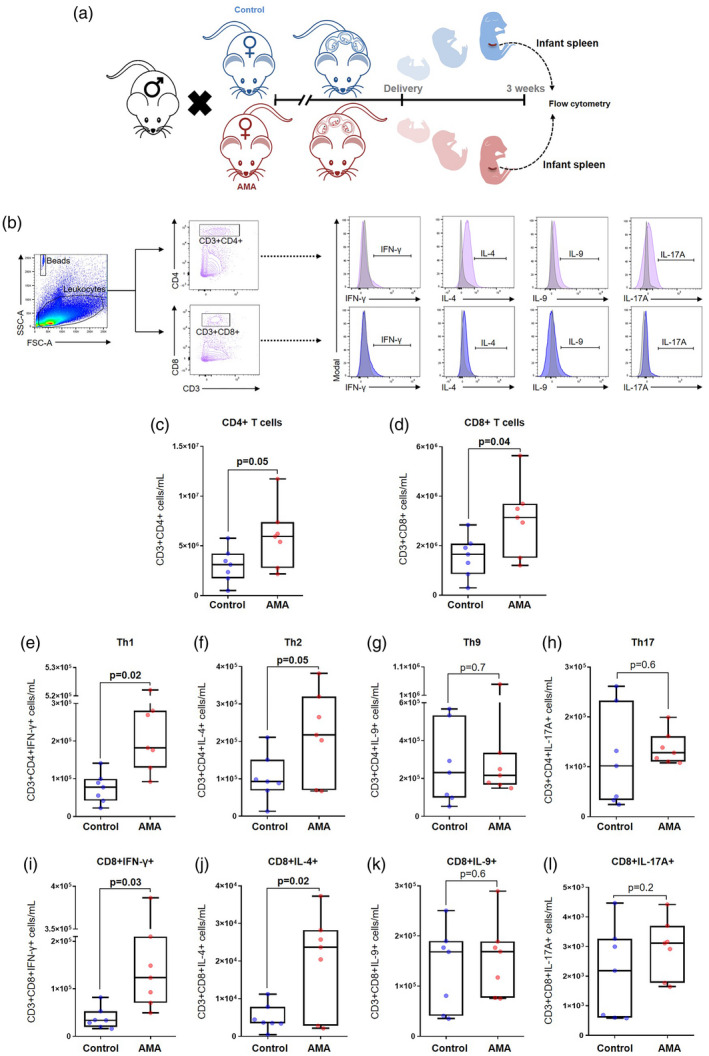
Immunophenotyping of T‐cell subsets in the spleen of infants from advanced maternal aged dams. (a) Experimental design of infant spleen collection from offspring of control and advanced maternal aged (AMA) dams. (b) Gating strategy used to quantify the T‐cell subsets in the infant spleen. Number of (c) CD4+ T cells, (d) CD8+ T cells, (e) T helper type 1 (Th1) cells, (f) Th2 cells, (g) Th9 cells, (h) Th17 cells, (i) CD8+ cells expressing IFN‐γ, (j) CD8+ cells expressing IL‐4, (k) CD8+ cells expressing IL‐9, and (l) CD8+ cells expressing IL‐17A (n = 7 each). Mid‐lines indicate medians, boxes indicate interquartile ranges and whiskers indicate minimum–maximum range. The P‐values were determined by an unpaired t‐test or a Mann–Whitney U‐test. Significant P‐values are shown in bold type.
In line with these findings, the numbers of splenic CD8+IFN‐γ+ T cells were significantly elevated in the offspring of AMA dams compared to controls (Fig. 5i), as were the numbers of splenic CD8+IL‐4+ T cells (Fig. 5j). There were no differences in the numbers of splenic CD8+IL‐9+ T cells (Fig. 5k) or in the numbers of splenic CD8+IL‐17A+ T cells (Fig. 5l) between infants from AMA dams and those from controls.
We also characterized splenic CD4+ Tregs from the infants of AMA and control dams (Fig. 6a). Consistent with the numbers of conventional T cells, the numbers of splenic CD4+ Treg cells were increased in infants from AMA dams compared to those from controls (Fig. 6b; P = 0·05). Moreover, the numbers of splenic CD8+CD25+FoxP3+ T cells were also elevated in infants from AMA dams compared to those from controls (Fig. 6c). These results suggest that the offspring from advanced‐age mothers who survive past the neonatal window undergo compensatory alterations in the T‐cell repertoire.
Fig. 6.
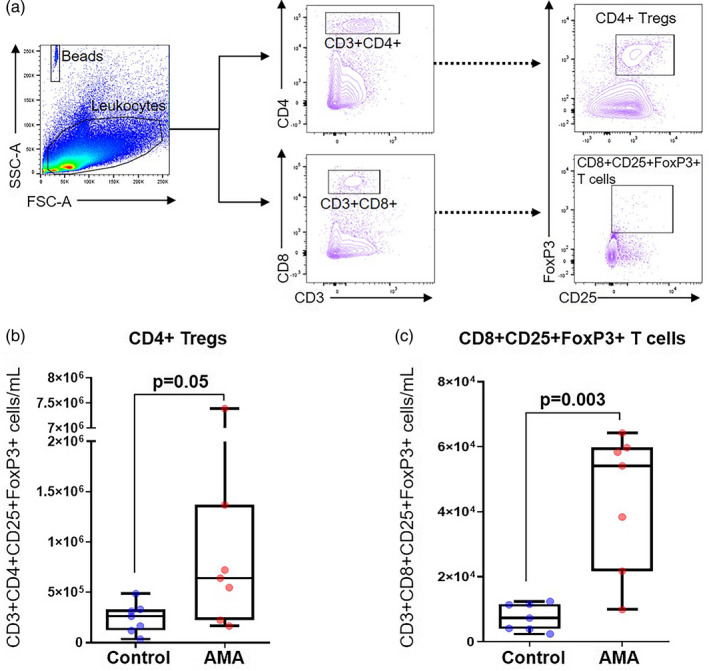
Immunophenotyping of regulatory T cells (Tregs) in the spleen of infants from advanced maternal aged (AMA) dams. (a) Gating strategy used to quantify the Tregs in the infant spleen. Number of (b) CD4+ Tregs and (c) CD8+CD25+FoxP3+ T cells (n = 7 each) in control and AMA dams. Mid‐lines indicate medians, boxes indicate interquartile ranges and whiskers indicate minimum–maximum range. The P‐values were determined by an unpaired t‐test or a Mann–Whitney U‐test. Significant P‐values are shown in bold type.
Advanced maternal age does not alter infant CD71+ erythroid cells
There is an increasing body of evidence reporting that neonatal innate and adaptive immunity depends on the critical immunomodulatory functions of CD71+ nucleated erythroid cells [119, 120, 121, 122, 123, 124]. Indeed, we have shown that CD71+ erythroid cells play a central role by modulating immune responses in neonates born to mothers who underwent the process of preterm or term labor [125, 126]. Therefore, we investigated the effect of AMA on the CD71+ erythroid cell population of the offspring at 1 and 3 weeks of age (Fig. 7a). The proportions of CD71+ erythroid cells in the offspring of AMA dams were unaffected at 1 week of age compared to those from controls (Fig. 7b). Similarly, CD71+ erythroid cells were unchanged at 3 weeks after birth (Fig. 7c). These findings show that AMA does not affect the proportion of CD71+ erythroid cells in the offspring; however, further studies are required to investigate whether AMA alters the functionality of these cells.
Fig. 7.
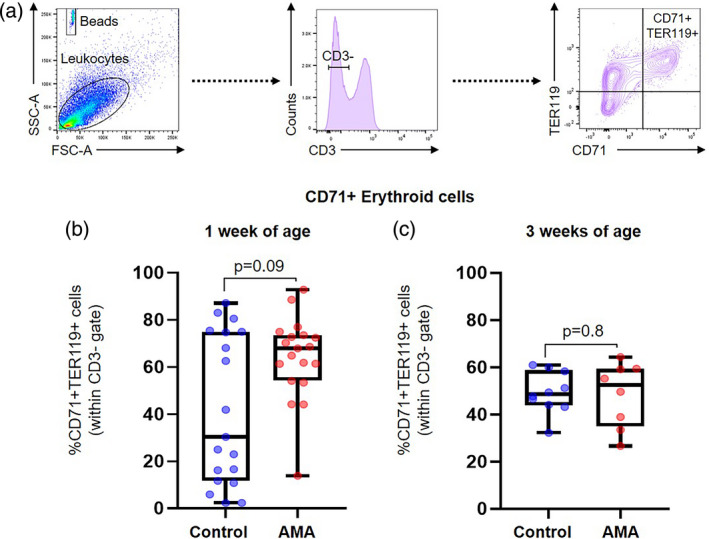
Immunophenotyping of CD71+ erythroid cells in the spleen of offspring from advanced maternal aged (AMA) dams. (a) Gating strategy used to quantify CD71+ erythroid cells. Proportion of CD71+ erythroid cells (b) in the neonatal spleen at 1 week of age and (c) in the infant spleen at 3 weeks of age (n = 8–19 each) in control and AMA groups. Mid‐lines indicate medians, boxes indicate interquartile ranges and whiskers indicate minimum–maximum range. The P‐values were determined by an unpaired t‐test or a Mann–Whitney U‐test. Significant P‐values are shown in bold type.
Discussion
The current study provides evidence that advanced maternal age: (1) impairs fertility, the process of labor, and the timing of delivery; (2) diminishes the number of specific proinflammatory T‐cell subsets (Th1, Th9, and CD8+IFN‐γ+) at the maternal–fetal interface prior to term parturition; (3) reduces the number of CD4+ Tregs, but not CD8+CD25+FoxP3+ T cells, at the maternal–fetal interface prior to parturition; (4) alters the growth and survival of the offspring in early life; (5) induces an expansion of IFN‐γ‐ and IL‐4‐producing CD4+ and CD8+ T cells, as well as CD4+ Tregs and CD8+CD25+FoxP3+ T cells, in the infant; and (6) does not alter the proportion of CD71+ erythroid cells in the offspring. Collectively, these findings provide a phenotypical characterization of the effects of advanced maternal age on T‐cell responses at the maternal–fetal interface prior to term labor and in the offspring.
In recent years, a growing body of evidence suggests a role for maternal T cells in the processes of labor: (1) T cells are actively recruited from the periphery into the decidual tissues through chemotactic processes during the onset of term labor [57, 97, 127]; (2) T cells, including exhausted T cells, are enriched at the human maternal–fetal interface prior to the onset of term labor [99, 128, 129, 130, 131, 132, 133, 134, 135]; (3) T‐cell exhaustion at the maternal–fetal interface is reversed by inflammatory mediators associated with term labor [99]; (4) activated T cells at the maternal–fetal interface express labor mediators such as tumor necrosis factor (TNF)‐α, IL‐1β, and matrix metalloproteinase (MMP)‐9 during the process of term labor [66]; (5) effector T cells expressing perforin are increased at the maternal–fetal interface of women with term labor compared to non‐labor controls [65]; (6) IL‐6, a cytokine that participates in the timing of parturition [136], controls decidual T‐cell subsets prior to term labor [98]; (7) activation of T cells by administration of an anti‐CD3 antibody induces the process of preterm labor [13]; (8) effector and activated T cells expressing granzyme B and perforin are enriched at the maternal–fetal interface of women who underwent spontaneous preterm labor and birth [65]; and (9) single‐cell RNA signatures of activated T cells precede term and preterm labor [69, 90]. Herein, we put forward evidence that advanced maternal age is associated with a pathological delay of the process of term labor, and this is associated with alterations in T‐cell subsets at the maternal–fetal interface.
The mechanisms whereby advanced maternal age induces dystocia and extended duration of labor may involve alterations in the proinflammatory milieu localized at the maternal–fetal interface. For example, we found that mothers of advanced age had fewer Th1 and Th9 cells in the decidual tissues. These T‐cell subsets have been previously reported at the maternal–fetal interface [98, 137, 138]. The differentiation of Th1 and Th9 cells results from stimulation with specific cytokines such as IL‐12/IFN‐γ [139, 140, 141] and IL‐4/transforming growth factor (TGF)‐β1 [108, 109, 142], respectively. Therefore, it is tempting to suggest that the decidual tissues of advanced‐age mothers are deficient in local cytokines required for the differentiation of the Th1 and Th9 subsets. Herein, we also report that advanced‐age mothers had fewer IFN‐γ‐ and IL‐4‐expressing CD8+ T cells at the maternal–fetal interface. This observation may also reflect defective inflammatory signaling pathways in the decidual tissues of advanced‐age mothers. Further investigation is required to elucidate the molecular mechanisms whereby advanced maternal age causes impaired T‐cell subset differentiation at the maternal–fetal interface prior to term labor.
CD4+ Tregs are an important subset of T cells that express CD25 and FoxP3 [143, 144, 145, 146]. These cells play a central role in immune tolerance by exhibiting suppressive activity towards both self‐ and non‐self‐antigens [147, 148, 149]. This suppressive activity is due largely to the expression of the transcription factor FoxP3 [144, 150]. CD4+ Tregs have been localized at the human [151, 152] and murine [67, 153, 154] maternal–fetal interface. These cells seem to play an important role in promoting maternal–fetal tolerance from early to mid‐pregnancy [153, 155, 156, 157]. However, this T‐cell subset has not been involved in the timing of parturition at term, as shown herein at the maternal–fetal interface of advanced‐age mothers. The cellular mechanisms (e.g. TGF‐β1 signaling) whereby advanced age causes a reduction in the number of CD4+ Tregs in the decidua are worthy of further research.
Our study provides a phenotypical characterization of the T‐cell subsets that are defective at the maternal–fetal interface of advanced‐age mothers. However, it is important to note that aging of the reproductive organs [158, 159, 160, 161, 162], as well as other pathological processes [163, 164, 165, 166], must be considered when studying the mechanisms whereby advanced maternal age causes prolonged labor. It is worth mentioning that the pathological processes involved in prolonged labor are confined to the intrauterine space, given that the alterations in T‐cell subsets were not observed in the lymphatic tissues.
In this study, we also report that advanced maternal age is associated with reduced early offspring survival, which is in tandem with our finding of reduced fetal weight. This is consistent with prior studies that have associated advanced maternal age in mice with decreased offspring body weight and life expectancy [167, 168, 169]. Future studies are warranted to determine whether this finding is due to postnatal factors such as maternal caring behaviors or differences in breast milk quantity and composition, or antenatal and perinatal factors that occur in utero. We also found that advanced maternal age alters T‐cell subsets, including CD4+ Tregs, without affecting other immunomodulatory cells (e.g. CD71+ erythroid cells) in infants. To our knowledge, this is the first demonstration that infants of mothers of advanced age have increased numbers of CD4+ and CD8+ T cells expressing IFN‐γ, IL‐4, and FoxP3. This rise may be due to a compensatory mechanism in response to the adverse/extended intrauterine environment [84, 85], which is reflected by the appropriate weight gain in infants at 3 weeks of age. A similar compensatory mechanism has also been observed in infants born to mothers who experienced chronic prenatal stress [170]. Nevertheless, the immunocompetence of pups born to dams of advanced age remains to be elucidated.
Conclusion
The data in the current study provide evidence that advanced maternal age impairs the process of labor and alters the T‐cell repertoire at the maternal–fetal interface prior to term labor. Additionally, we show that advanced maternal age is associated with adverse consequences for the offspring, as demonstrated by affected growth patterns and T‐cell responses. Together, these findings represent the first characterization of the effect of advanced maternal age on the main cellular branch of adaptive immunity, T cells, at the maternal–fetal interface prior to term labor and in the offspring. These findings provide insight into the immune mechanisms dysregulated in the pathological process of delayed labor and, more importantly, suggest that infants born to mothers of advanced age may display impaired T‐cell immunity.
Disclosures
The authors have no financial conflicts of interest.
Author contributions
D. L., V. G‐F., D. M. and Y. X. performed research and analyzed and interpreted data. R. R. interpreted data and provided guidance in the experimental design. A. S. analyzed and interpreted data. S. S. H. provided intellectual input. N. G.‐L. designed research, interpreted data, and provided supervision throughout the study. All authors participated in the writing of the manuscript.
Supporting information
Fig. S1. Immunophenotyping of the T‐cell subsets in the uterine draining lymph nodes (ULN) of advanced maternal aged (AMA) dams. Number of (a) CD4+ T cells, (b) CD8+ T cells, (c) Th1 cells, (d) Th2 cells, (e) Th9 cells, (f) Th17 cells, (g) CD8+ cells expressing IFNγ, (h) CD8+ cells expressing IL‐4, (i) CD8+ cells expressing IL‐9, and (j) CD8+ cells expressing IL‐17A (n = 6‐10 each) in control and AMA dams. Mid‐lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min‐max range. The p values were determined by an unpaired t test or a Mann‐Whitney U test.
Fig. S2. Immunophenotyping of the T‐cell subsets in the spleen of advanced maternal aged (AMA) dams. Number of (a) CD4+ T cells, (b) CD8+ T cells, (c) Th1 cells, (d) Th2 cells, (e) Th9 cells, (f) Th17 cells, (g) CD8+ cells expressing IFNγ, (h) CD8+ cells expressing IL‐4, (i) CD8+ cells expressing IL‐9, and (j) CD8+ cells expressing IL‐17A (n = 7‐10 each) in control and AMA dams. Mid‐lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min‐max range. The p values were determined by an unpaired t test or a Mann‐Whitney U test.
Fig. S3. Immunophenotyping of regulatory T cells (Tregs) in the uterine draining lymph nodes (ULN) and spleen of advanced maternal aged (AMA) and control dams. Number of (a) CD4+ Tregs and (b) CD8+CD25+FoxP3+ T cells in the ULN (n = 6‐10 each). Number of (c) CD4+ Tregs and (d) CD8+CD25+FoxP3+ T cells in the spleen (n = 7‐10 each). Mid‐lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min‐max range. The p values were determined by an unpaired t test or a Mann‐Whitney U test.
Table S1. Antibodies used for immunophenotyping.
Acknowledgements
This research was supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health and the Perinatology Research Branch, Division of Obstetrics and Maternal–Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with federal funds from NICHD/NIH/DHHS under Contract no. HHSN275201300006C. R. R. has contributed to this work as part of his official duties as an employee of the United States Federal Government.
References
- 1. United Nations . World Fertility Report 2015. Contract no.: ST/ESA/SER.A/415. New York, NY: United Nations, Department of Economic and Social Affairs, Population Division; 2017. [Google Scholar]
- 2. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep 2018; 67:1–55. [PubMed] [Google Scholar]
- 3. Duckitt K, Harrington D. Risk factors for pre‐eclampsia at antenatal booking: systematic review of controlled studies. BMJ 2005; 330:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol 2005; 105:1410–8. [DOI] [PubMed] [Google Scholar]
- 5. Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol 2013; 42:634–43. [DOI] [PubMed] [Google Scholar]
- 6. Berkowitz GS, Skovron ML, Lapinski RH, Berkowitz RL. Delayed childbearing and the outcome of pregnancy. N Engl J Med 1990; 322:659–64. [DOI] [PubMed] [Google Scholar]
- 7. Cleary‐Goldman J, Malone FD, Vidaver J et al Impact of maternal age on obstetric outcome. Obstet Gynecol 2005; 105:983–90. [DOI] [PubMed] [Google Scholar]
- 8. Treacy A, Robson M, O’Herlihy C. Dystocia increases with advancing maternal age. Am J Obstet Gynecol 2006; 195:760–3. [DOI] [PubMed] [Google Scholar]
- 9. Luke B, Brown MB. Elevated risks of pregnancy complications and adverse outcomes with increasing maternal age. Hum Reprod 2007; 22:1264–72. [DOI] [PubMed] [Google Scholar]
- 10. Kozinszky Z, Orvos H, Zoboki T et al Risk factors for cesarean section of primiparous women aged over 35 years. Acta Obstet Gynecol Scand 2002; 81:313–6. [DOI] [PubMed] [Google Scholar]
- 11. Lin HC, Sheen TC, Tang CH, Kao S. Association between maternal age and the likelihood of a cesarean section: a population‐based multivariate logistic regression analysis. Acta Obstet Gynecol Scand 2004; 83:1178–83. [DOI] [PubMed] [Google Scholar]
- 12. Janoudi G, Kelly S, Yasseen A, Hamam H, Moretti F, Walker M. Factors associated with increased rates of caesarean section in women of advanced maternal age. J Obstet Gynaecol Can 2015; 37:517–26. [DOI] [PubMed] [Google Scholar]
- 13. Oliveira FC Jr, Costa ML, Cecatti JG, Pinto e Silva JL, Surita FG. Maternal morbidity and near miss associated with maternal age: the innovative approach of the 2006 Brazilian demographic health survey. Clinics 2013; 68:922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laopaiboon M, Lumbiganon P, Intarut N et al Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG 2014; 121(Suppl 1):49–56. [DOI] [PubMed] [Google Scholar]
- 15. Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol 1998; 179:80–6. [DOI] [PubMed] [Google Scholar]
- 16. Naccasha N, Gervasi MT, Chaiworapongsa T et al Phenotypic and metabolic characteristics of monocytes and granulocytes in normal pregnancy and maternal infection. Am J Obstet Gynecol 2001; 185:1118–23. [DOI] [PubMed] [Google Scholar]
- 17. Unal ER, Cierny JT, Roedner C, Newman R, Goetzl L. Maternal inflammation in spontaneous term labor. Am J Obstet Gynecol 2011; 204:223.e1–5. [DOI] [PubMed] [Google Scholar]
- 18. Cierny JT, Unal ER, Flood P et al Maternal inflammatory markers and term labor performance. Am J Obstet Gynecol 2014;210:447.e1–6. [DOI] [PubMed] [Google Scholar]
- 19. Neal JL, Lamp JM, Lowe NK, Gillespie SL, Sinnott LT, McCarthy DO. Differences in inflammatory markers between nulliparous women admitted to hospitals in preactive vs active labor. Am J Obstet Gynecol 2015; 212:68.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haddad R, Tromp G, Kuivaniemi H et al Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006; 195:394.e1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nhan‐Chang CL, Romero R, Tarca AL et al Characterization of the transcriptome of chorioamniotic membranes at the site of rupture in spontaneous labor at term. Am J Obstet Gynecol 2010; 202:462.e1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hassan SS, Romero R, Haddad R et al The transcriptome of the uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2006; 195:778–86. [DOI] [PubMed] [Google Scholar]
- 23. Hassan SS, Romero R, Tarca AL et al Signature pathways identified from gene expression profiles in the human uterine cervix before and after spontaneous term parturition. Am J Obstet Gynecol 2007; 197:250.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mittal P, Romero R, Tarca AL et al Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med 2010; 38:617–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaemsaithong P, Madan I, Romero R et al Characterization of the myometrial transcriptome in women with an arrest of dilatation during labor. J Perinat Med 2013; 41:665–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romero R, Tarca AL, Chaemsaithong P et al Transcriptome interrogation of human myometrium identifies differentially expressed sense‐antisense pairs of protein‐coding and long non‐coding RNA genes in spontaneous labor at term. J Matern Fetal Neonatal Med 2014; 27:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dudley DJ, Collmer D, Mitchell MD, Trautman MS. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig 1996; 3:328–35. [PubMed] [Google Scholar]
- 28. Ammala M, Nyman T, Salmi A, Rutanen EM. The interleukin‐1 system in gestational tissues at term: effect of labour. Placenta 1997; 18:717–23. [DOI] [PubMed] [Google Scholar]
- 29. Stephen GL, Lui S, Hamilton SA et al Transcriptomic profiling of human choriodecidua during term labor: inflammation as a key driver of labor. Am J Reprod Immunol 2015; 73:36–55. [DOI] [PubMed] [Google Scholar]
- 30. Bukowski R, Sadovsky Y, Goodarzi H et al Onset of human preterm and term birth is related to unique inflammatory transcriptome profiles at the maternal fetal interface. PeerJ 2017; 5:e3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arenas‐Hernandez M, Gomez‐Lopez N, Garcia‐Flores V et al Choriodecidual leukocytes display a unique gene expression signature in spontaneous labor at term. Genes Immun 2019; 20:56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liggins CG. Cervical ripening as an inflammatory reaction In: Elwood DA, Andersson ABM, eds. Cervix in pregnancy and labour. Edinburgh: Churchill Livingstone; 1981:1–9. [Google Scholar]
- 33. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007; 65:S194–202. [DOI] [PubMed] [Google Scholar]
- 34. Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth 2007; 7(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Norwitz ER, Bonney EA, Snegovskikh VV et al Molecular regulation of parturition: the role of the decidual clock. Cold Spring Harb Perspect Med 2015; 5 pii: a023143 10.1101/cshperspect.a023143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bokstrom H, Brannstrom M, Alexandersson M, Norstrom A. Leukocyte subpopulations in the human uterine cervical stroma at early and term pregnancy. Hum Reprod 1997; 12:586–90. [DOI] [PubMed] [Google Scholar]
- 37. Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod 1999; 61:879–83. [DOI] [PubMed] [Google Scholar]
- 38. Young A, Thomson AJ, Ledingham M, Jordan F, Greer IA, Norman JE. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod 2002; 66:445–9. [DOI] [PubMed] [Google Scholar]
- 39. Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol 2002; 57:217–24. [DOI] [PubMed] [Google Scholar]
- 40. Osman I, Young A, Ledingham MA et al Leukocyte density and pro‐inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003; 9:41–5. [DOI] [PubMed] [Google Scholar]
- 41. Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig 2003; 10:323–38. [DOI] [PubMed] [Google Scholar]
- 42. Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol 2005; 66:161–73. [DOI] [PubMed] [Google Scholar]
- 43. Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod 2008; 78:438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol 2009; 182:2700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yellon SM, Oshiro BT, Chhaya TY et al Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod 2011; 85:498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, Yellon SM. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod 2011; 84:587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Payne KJ, Clyde LA, Weldon AJ, Milford TA, Yellon SM. Residency and activation of myeloid cells during remodeling of the prepartum murine cervix. Biol Reprod 2012; 87:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Myers DA. The recruitment and activation of leukocytes into the immune cervix: further support that cervical remodeling involves an immune and inflammatory mechanism. Biol Reprod 2012; 87:107. [DOI] [PubMed] [Google Scholar]
- 49. Thomson AJ, Telfer JF, Young A et al Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 1999; 14:229–36. [PubMed] [Google Scholar]
- 50. Shynlova O, Tsui P, Dorogin A, Lye SJ. Monocyte chemoattractant protein‐1 (CCL‐2) integrates mechanical and endocrine signals that mediate term and preterm labor. J Immunol 2008; 181:1470–9. [DOI] [PubMed] [Google Scholar]
- 51. Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol 2009; 144(Suppl 1):S2–10. [DOI] [PubMed] [Google Scholar]
- 52. Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci 2013; 20:154–67. [DOI] [PubMed] [Google Scholar]
- 53. Fidel PL Jr, Romero R, Ramirez M et al Interleukin‐1 receptor antagonist (IL‐1ra) production by human amnion, chorion, and decidua. Am J Reprod Immunol 1994; 32:1–7. [DOI] [PubMed] [Google Scholar]
- 54. Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol 1999; 181:1530–6. [DOI] [PubMed] [Google Scholar]
- 55. Lonergan M, Aponso D, Marvin KW et al Tumor necrosis factor‐related apoptosis‐inducing ligand (TRAIL), TRAIL receptors, and the soluble receptor osteoprotegerin in human gestational membranes and amniotic fluid during pregnancy and labor at term and preterm. J Clin Endocrinol Metab 2003; 88:3835–44. [DOI] [PubMed] [Google Scholar]
- 56. Esplin MS, Peltier MR, Hamblin S et al Monocyte chemotactic protein‐1 expression is increased in human gestational tissues during term and preterm labor. Placenta 2005; 26:661–71. [DOI] [PubMed] [Google Scholar]
- 57. Gomez‐Lopez N, Estrada‐Gutierrez G, Jimenez‐Zamudio L, Vega‐Sanchez R, Vadillo‐Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol 2009; 80:122–31. [DOI] [PubMed] [Google Scholar]
- 58. Gomez‐Lopez N, Vadillo‐Perez L, Nessim S, Olson DM, Vadillo‐Ortega F. Choriodecidua and amnion exhibit selective leukocyte chemotaxis during term human labor. Am J Obstet Gynecol 2011; 204:364.e9–16. [DOI] [PubMed] [Google Scholar]
- 59. Hadley EE, Sheller‐Miller S, Saade G et al Amnion epithelial cell‐derived exosomes induce inflammatory changes in uterine cells. Am J Obstet Gynecol 2018; 219:478.e1–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vince GS, Starkey PM, Jackson MC, Sargent IL, Redman CW. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods 1990; 132:181–9. [DOI] [PubMed] [Google Scholar]
- 61. Gomez‐Lopez N, Guilbert LJ, Olson DM. Invasion of the leukocytes into the fetal‐maternal interface during pregnancy. J Leukoc Biol 2010; 88:625–33. [DOI] [PubMed] [Google Scholar]
- 62. Hamilton S, Oomomian Y, Stephen G et al Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod 2012; 86:39. [DOI] [PubMed] [Google Scholar]
- 63. Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLOS ONE 2013; 8:e56946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu Y, Romero R, Miller D et al An M1‐like macrophage polarization in decidual tissue during spontaneous preterm labor that is attenuated by rosiglitazone treatment. J Immunol 2016; 196:2476–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Arenas‐Hernandez M, Romero R, Xu Y et al Effector and activated T cells induce preterm labor and birth that is prevented by treatment with progesterone. J Immunol 2019; 202:2585–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gomez‐Lopez N, Vega‐Sanchez R, Castillo‐Castrejon M, Romero R, Cubeiro‐Arreola K, Vadillo‐Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol 2013; 69:212–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arenas‐Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, Gomez‐Lopez N. An imbalance between innate and adaptive immune cells at the maternal–fetal interface occurs prior to endotoxin‐induced preterm birth. Cell Mol Immunol 2016; 13:462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gomez‐Lopez N, StLouis D, Lehr MA, Sanchez‐Rodriguez EN, Arenas‐Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol 2014; 11:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pique‐Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia‐Flores V, et al Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife 2019; 8 pii: e52004 10.7554/eLife.52004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Flood TM, Brink SJ, Gleason RE. Increased incidence of type I diabetes in children of older mothers. Diabetes Care 1982; 5:571–3. [DOI] [PubMed] [Google Scholar]
- 71. Metcalfe MA, Baum JD. Family characteristics and insulin dependent diabetes. Arch Dis Child 1992; 67:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bingley PJ, Douek IF, Rogers CA, Gale EA. Influence of maternal age at delivery and birth order on risk of type 1 diabetes in childhood: prospective population based family study. Bart’s–Oxford Family Study Group. BMJ 2000; 321:420–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dioun AF, Harris SK, Hibberd PL. Is maternal age at delivery related to childhood food allergy? Pediatr Allergy Immunol 2003; 14:307–11. [DOI] [PubMed] [Google Scholar]
- 74. Tarin JJ, Vidal E, Perez‐Hoyos S, Cano A, Balasch J. Delayed motherhood increases the probability of sons to be infertile. J Assist Reprod Genet 2001; 18:650–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Smits LJ, Willemsen WN, Zielhuis GA, Jongbloet PH. Conditions at conception and risk of menstrual disorders. Epidemiology 1997; 8:524–9. [DOI] [PubMed] [Google Scholar]
- 76. Yip BH, Pawitan Y, Czene K. Parental age and risk of childhood cancers: a population‐based cohort study from Sweden. Int J Epidemiol 2006; 35:1495–503. [DOI] [PubMed] [Google Scholar]
- 77. Lopez‐Castroman J, Gomez DD, Belloso JJ et al Differences in maternal and paternal age between schizophrenia and other psychiatric disorders. Schizophr Res 2010; 116:184–90. [DOI] [PubMed] [Google Scholar]
- 78. Sandin S, Hultman CM, Kolevzon A, Gross R, MacCabe JH, Reichenberg A. Advancing maternal age is associated with increasing risk for autism: a review and meta‐analysis. J Am Acad Child Adolesc Psychiatry 2012; 51:477–86.e1. [DOI] [PubMed] [Google Scholar]
- 79. Li S, Hua J, Hong H, Wang Y, Zhang J. Interpregnancy interval, maternal age, and offspring's BMI and blood pressure at 7 years of age. J Hum Hypertens 2018; 32:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Imterat M, Wainstock T, Sheiner E, Kapelushnik J, Fischer L, Walfisch A. Advanced maternal age during pregnancy and the risk for malignant morbidity in the childhood. Eur J Pediatr 2018; 177:879–86. [DOI] [PubMed] [Google Scholar]
- 81. Rios L, Vasquez L, Oscanoa M, Maza I, Geronimo J. Advancing parental age and risk of solid tumors in children: a case–control study in peru. J Oncol 2018; 2018:3924635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kollias C, Dimitrakopoulos S, Xenaki LA, Stefanis N, Papageorgiou C. Evidence of advanced parental age linked to sporadic schizophrenia. Psychiatriki 2019; 30:24–31. [DOI] [PubMed] [Google Scholar]
- 83. Tarin JJ, Garcia‐Perez MA, Cano A. Potential risks to offspring of intrauterine exposure to maternal age‐related obstetric complications. Reprod Fertil Dev 2017; 29:1468–76. [DOI] [PubMed] [Google Scholar]
- 84. Langley‐Evans SC. Developmental programming of health and disease. Proc Nutr Soc 2006; 65:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gluckman PD, Hanson MA, Buklijas T. A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis 2010; 1:6–18. [DOI] [PubMed] [Google Scholar]
- 86. Chen T, Liu HX, Yan HY, Wu DM, Ping J. Developmental origins of inflammatory and immune diseases. Mol Hum Reprod 2016; 22:858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shirasuna K, Iwata H. Effect of aging on the female reproductive function. Contracept Reprod Med 2017; 2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aghaeepour N, Ganio EA, McIlwain D et al An immune clock of human pregnancy. Sci Immunol 2017; 2 pii: eaan2946 10.1126/sciimmunol.aan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aghaeepour N, Lehallier B, Baca Q et al A proteomic clock of human pregnancy. Am J Obstet Gynecol 2018; 218:347.e1 –e14. [DOI] [PubMed] [Google Scholar]
- 90. Tarca AL, Romero R, Xu Z et al Targeted expression profiling by RNA‐Seq improves detection of cellular dynamics during pregnancy and identifies a role for T cells in term parturition. Sci Rep 2019; 9:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gomez‐Lopez N, Romero R, Hassan SS et al The cellular transcriptome in the maternal circulation during normal pregnancy: a longitudinal study. Front Immunol 2019; 10:2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Arenas‐Hernandez M, Sanchez‐Rodriguez EN, Mial TN, Robertson SA, Gomez‐Lopez N. Isolation of leukocytes from the murine tissues at the maternal–fetal interface. J Vis Exp 2015; 99:e52866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Behringer R, Gertsenstein M, Nagy KV, Nagy A. Manipulating the mouse embryo: a laboratory manual. Cold Spring Habor, NY: Cold Spring Habor Laboratory Press; 2014. [Google Scholar]
- 94. St Louis D, Romero R, Plazyo O et al Invariant NKT cell activation induces late preterm birth that is attenuated by rosiglitazone. J Immunol 2016; 196:1044–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Garcia‐Flores V, Romero R, Miller D et al Inflammation‐induced adverse pregnancy and neonatal outcomes can be improved by the immunomodulatory peptide exendin‐4. Front Immunol 2018; 9:1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sengupta P. A scientific review of age determination for a laboratory rat: how old is it in comparison with human age. Biomed Int 2011; 2:81–9. [Google Scholar]
- 97. Gomez‐Lopez N, Vadillo‐Perez L, Hernandez‐Carbajal A, Godines‐Enriquez M, Olson DM, Vadillo‐Ortega F. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol 2011; 205:235.e15–24. [DOI] [PubMed] [Google Scholar]
- 98. Gomez‐Lopez N, Olson DM, Robertson SA. Interleukin‐6 controls uterine Th9 cells and CD8(+) T regulatory cells to accelerate parturition in mice. Immunol Cell Biol 2016; 94:79–89. [DOI] [PubMed] [Google Scholar]
- 99. Slutsky R, Romero R, Xu Y et al Exhausted and senescent t cells at the maternal–fetal interface in preterm and term labor. J Immunol Res 2019; 2019:3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, et al The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 2010; 23:1000–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lee J, Kim JS, Park JW et al Chronic chorioamnionitis is the most common placental lesion in late preterm birth. Placenta 2013; 34:681–9. [DOI] [PubMed] [Google Scholar]
- 102. Kim CJ, Romero R, Chaemsaithong P, Kim JS. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 2015; 213(Suppl 4):S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gomez‐Lopez N, Romero R, Arenas‐Hernandez M et al In vivo T‐cell activation by a monoclonal alphaCD3epsilon antibody induces preterm labor and birth. Am J Reprod Immunol 2016; 76:386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986; 136:2348–57. [PubMed] [Google Scholar]
- 105. Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989; 7:145–73. [DOI] [PubMed] [Google Scholar]
- 106. Renauld JC, Goethals A, Houssiau F, Merz H, Van Roost E, Van Snick J. Human P40/IL‐9. Expression in activated CD4+ T cells, genomic organization, and comparison with the mouse gene. J Immunol 1990; 144:4235–41. [PubMed] [Google Scholar]
- 107. Schmitt E, Germann T, Goedert S et al IL‐9 production of naive CD4+ T cells depends on IL‐2, is synergistically enhanced by a combination of TGF‐beta and IL‐4, and is inhibited by IFN‐gamma. J Immunol 1994; 153:3989–96. [PubMed] [Google Scholar]
- 108. Dardalhon V, Awasthi A, Kwon H et al IL‐4 inhibits TGF‐beta‐induced Foxp3+ T cells and together with TGF‐beta, generates IL‐9+ IL‐10+ Foxp3(–) effector T cells. Nat Immunol 2008; 9:1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Veldhoen M, Uyttenhove C, van Snick J et al Transforming growth factor‐beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9‐producing subset. Nat Immunol 2008; 9:1341–6. [DOI] [PubMed] [Google Scholar]
- 110. Park H, Li Z, Yang XO et al A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kisielewicz A, Schaier M, Schmitt E et al A distinct subset of HLA‐DR+‐regulatory T cells is involved in the induction of preterm labor during pregnancy and in the induction of organ rejection after transplantation. Clin Immunol 2010; 137:209–20. [DOI] [PubMed] [Google Scholar]
- 112. Xiong H, Zhou C, Qi G. Proportional changes of CD4+CD25+Foxp3+ regulatory T cells in maternal peripheral blood during pregnancy and labor at term and preterm. Clin Invest Med 2010; 33:E422. [DOI] [PubMed] [Google Scholar]
- 113. Steinborn A, Schmitt E, Kisielewicz A et al Pregnancy‐associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol 2012; 167:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T‐cell pool. Immunol Cell Biol 2012; 90:935–44. [DOI] [PubMed] [Google Scholar]
- 115. Gomez‐Lopez N, Laresgoiti‐Servitje E. T regulatory cells: regulating both term and preterm labor? Immunol Cell Biol 2012; 90:919–20. [DOI] [PubMed] [Google Scholar]
- 116. Haines CJ, Rogers MS, Leung DH. Neonatal outcome and its relationship with maternal age. Aust NZ J Obstet Gynaecol 1991; 31:209–12. [DOI] [PubMed] [Google Scholar]
- 117. Bahtiyar MO, Funai EF, Rosenberg V et al Stillbirth at term in women of advanced maternal age in the United States: when could the antenatal testing be initiated? Am J Perinatol 2008; 25:301–4. [DOI] [PubMed] [Google Scholar]
- 118. Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta‐analysis. PLOS ONE 2017; 12:e0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rincon MR, Oppenheimer K, Bonney EA. Selective accumulation of Th2‐skewing immature erythroid cells in developing neonatal mouse spleen. Int J Biol Sci 2012; 8:719–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Elahi S, Ertelt JM, Kinder JM et al Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013; 504:158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Elahi S. New insight into an old concept: role of immature erythroid cells in immune pathogenesis of neonatal infection. Front Immunol 2014; 5:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Namdar A, Koleva P, Shahbaz S, Strom S, Gerdts V, Elahi S. CD71(+) erythroid suppressor cells impair adaptive immunity against Bordetella pertussis. Sci Rep 2017; 7:7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Dunsmore G, Bozorgmehr N, Delyea C, Koleva P, Namdar A, Elahi S. Erythroid suppressor cells compromise neonatal immune response against bordetella pertussis. J Immunol 2017; 199:2081–95. [DOI] [PubMed] [Google Scholar]
- 124. Shahbaz S, Bozorgmehr N, Koleva P et al CD71+VISTA+ erythroid cells promote the development and function of regulatory T cells through TGF‐beta. PLOS Biol 2018; 16:e2006649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gomez‐Lopez N, Romero R, Xu Y et al Umbilical cord CD71+ erythroid cells are reduced in neonates born to women in spontaneous preterm labor. Am J Reprod Immunol 2016; 76:280–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Miller D, Romero R, Unkel R et al CD71+ erythroid cells from neonates born to women with preterm labor regulate cytokine and cellular responses. J Leukoc Biol 2018; 103:761–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gomez‐Lopez N, Hernandez‐Santiago S, Lobb AP, Olson DM, Vadillo‐Ortega F. Normal and premature rupture of fetal membranes at term delivery differ in regional chemotactic activity and related chemokine/cytokine production. Reprod Sci 2013; 20:276–84. [DOI] [PubMed] [Google Scholar]
- 128. Sindram‐Trujillo A, Scherjon S, Kanhai H, Roelen D, Claas F. Increased T‐cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol 2003; 49:261–8. [DOI] [PubMed] [Google Scholar]
- 129. Sindram‐Trujillo AP, Scherjon SA, van Hulst‐van Miert PP, Kanhai HH, Roelen DL, Claas FH. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol 2004; 62:125–37. [DOI] [PubMed] [Google Scholar]
- 130. Tilburgs T, Roelen DL, van der Mast BJ et al Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(–) T‐cells in decidua and maternal blood during human pregnancy. Placenta 2006; 27(Suppl A):S47–53. [DOI] [PubMed] [Google Scholar]
- 131. Tilburgs T, Scherjon SA, Roelen DL, Claas FH. Decidual CD8+CD28‐ T cells express CD103 but not perforin. Hum Immunol 2009; 70:96–100. [DOI] [PubMed] [Google Scholar]
- 132. Tilburgs T, van der Mast BJ, Nagtzaam NM, Roelen DL, Scherjon SA, Claas FH. Expression of NK cell receptors on decidual T cells in human pregnancy. J Reprod Immunol 2009; 80:22–32. [DOI] [PubMed] [Google Scholar]
- 133. Tilburgs T, Schonkeren D, Eikmans M et al Human decidual tissue contains differentiated CD8+ effector‐memory T cells with unique properties. J Immunol 2010; 185:4470–7. [DOI] [PubMed] [Google Scholar]
- 134. Powell RM, Lissauer D, Tamblyn J et al Decidual T cells exhibit a highly differentiated phenotype and demonstrate potential fetal specificity and a strong transcriptional response to IFN. J Immunol 2017; 199:3406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. van der Zwan A, Bi K, Norwitz ER et al Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci USA 2018; 115:385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Robertson SA, Christiaens I, Dorian CL et al Interleukin‐6 is an essential determinant of on‐time parturition in the mouse. Endocrinology 2010; 151:3996–4006. [DOI] [PubMed] [Google Scholar]
- 137. Saito S, Tsukaguchi N, Hasegawa T, Michimata T, Tsuda H, Narita N. Distribution of Th1, Th2, and Th0 and the Th1/Th2 cell ratios in human peripheral and endometrial T cells. Am J Reprod Immunol 1999; 42:240–5. [DOI] [PubMed] [Google Scholar]
- 138. Saito S. Cytokine network at the feto–maternal interface. J Reprod Immunol 2000; 47:87–103. [DOI] [PubMed] [Google Scholar]
- 139. Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL‐12 produced by Listeria‐induced macrophages. Science 1993; 260:547–9. [DOI] [PubMed] [Google Scholar]
- 140. Afkarian M, Sedy JR, Yang J et al T‐bet is a STAT1‐induced regulator of IL‐12R expression in naive CD4+ T cells. Nat Immunol 2002; 3:549–57. [DOI] [PubMed] [Google Scholar]
- 141. Trinchieri G, Pflanz S, Kastelein RA. The IL‐12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 2003; 19:641–4. [DOI] [PubMed] [Google Scholar]
- 142. Kaplan MH. Th9 cells: differentiation and disease. Immunol Rev 2013; 252:104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Itoh M, Takahashi T, Sakaguchi N et al Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self‐tolerance. J Immunol 1999; 162:5317–26. [PubMed] [Google Scholar]
- 144. Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057–61. [DOI] [PubMed] [Google Scholar]
- 145. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330–6. [DOI] [PubMed] [Google Scholar]
- 146. Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4:337–42. [DOI] [PubMed] [Google Scholar]
- 147. Fowell D, Mason D. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+ T cell subset that inhibits this autoimmune potential. J Exp Med 1993; 177:627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell‐mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol 2002; 32:1282–91. [DOI] [PubMed] [Google Scholar]
- 149. Sakaguchi S. Naturally arising Foxp3‐expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non‐self. Nat Immunol 2005; 6:345–52. [DOI] [PubMed] [Google Scholar]
- 150. Walker MR, Kasprowicz DJ, Gersuk VH et al Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J Clin Invest 2003; 112:1437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Heikkinen J, Mottonen M, Alanen A, Lassila O. Phenotypic characterization of regulatory T cells in the human decidua. Clin Exp Immunol 2004; 136:373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 2004; 10:347–53. [DOI] [PubMed] [Google Scholar]
- 153. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5:266–71. [DOI] [PubMed] [Google Scholar]
- 154. Furcron AE, Romero R, Plazyo O et al Vaginal progesterone, but not 17alpha‐hydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal–fetal interface. Am J Obstet Gynecol 2015; 213:846.e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kahn DA, Baltimore D. Pregnancy induces a fetal antigen‐specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci USA 2010; 107:9299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal–fetal conflict. Cell 2012; 150:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012; 490:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Cano F, Simon C, Remohi J, Pellicer A. Effect of aging on the female reproductive system: evidence for a role of uterine senescence in the decline in female fecundity. Fertil Steril 1995; 64:584–9. [DOI] [PubMed] [Google Scholar]
- 159. Pellicer A, Simon C, Remohi J. Effects of aging on the female reproductive system. Hum Reprod 1995; 10(Suppl 2):77–83. [DOI] [PubMed] [Google Scholar]
- 160. Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature 1998; 392:807–11. [DOI] [PubMed] [Google Scholar]
- 161. Main DM, Main EK, Moore DH II. The relationship between maternal age and uterine dysfunction: a continuous effect throughout reproductive life. Am J Obstet Gynecol 2000; 182:1312–20. [DOI] [PubMed] [Google Scholar]
- 162. Elmes M, Szyszka A, Pauliat C et al Maternal age effects on myometrial expression of contractile proteins, uterine gene expression, and contractile activity during labor in the rat. Physiol Rep 2015; 3 pii: e12305 10.14814/phy2.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Friedman CI, Danforth DR, Herbosa‐Encarnacion C, Arbogast L, Alak BM, Seifer DB. Follicular fluid vascular endothelial growth factor concentrations are elevated in women of advanced reproductive age undergoing ovulation induction. Fertil Steril 1997; 68:607–12. [DOI] [PubMed] [Google Scholar]
- 164. Jiang X, Yan J, Sheng Y, Sun M, Cui L, Chen ZJ. Low anti‐Mullerian hormone concentration is associated with increased risk of embryonic aneuploidy in women of advanced age. Reprod Biomed Online 2018; 37:178–83. [DOI] [PubMed] [Google Scholar]
- 165. Napso T, Hung YP, Davidge ST, Care AS, Sferruzzi‐Perri AN. Advanced maternal age compromises fetal growth and induces sex‐specific changes in placental phenotype in rats. Sci Rep 2019; 9:16916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Dogan B, Karaer A, Tuncay G, Tecellioglu N, Mumcu A. High‐resolution (1)H‐NMR spectroscopy indicates variations in metabolomics profile of follicular fluid from women with advanced maternal age. J Assist Reprod Genet 2020; 37:321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Wang MH, vom Saal FS. Maternal age and traits in offspring. Nature 2000; 407:469–70. [DOI] [PubMed] [Google Scholar]
- 168. Tarin JJ, Gomez‐Piquer V, Rausell F, Navarro S, Hermenegildo C, Cano A. Delayed motherhood decreases life expectancy of mouse offspring. Biol Reprod 2005; 72:1336–43. [DOI] [PubMed] [Google Scholar]
- 169. Carnes BA, Riesch R, Schlupp I. The delayed impact of parental age on offspring mortality in mice. J Gerontol A Biol Sci Med Sci 2012; 67:351–7. [DOI] [PubMed] [Google Scholar]
- 170. Garcia‐Flores V, Romero R, Furcron AE et al Prenatal maternal stress causes preterm birth and affects neonatal adaptive immunity in mice. Front Immunol 2020; 11:254 10.3389/fimmu.2020.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Immunophenotyping of the T‐cell subsets in the uterine draining lymph nodes (ULN) of advanced maternal aged (AMA) dams. Number of (a) CD4+ T cells, (b) CD8+ T cells, (c) Th1 cells, (d) Th2 cells, (e) Th9 cells, (f) Th17 cells, (g) CD8+ cells expressing IFNγ, (h) CD8+ cells expressing IL‐4, (i) CD8+ cells expressing IL‐9, and (j) CD8+ cells expressing IL‐17A (n = 6‐10 each) in control and AMA dams. Mid‐lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min‐max range. The p values were determined by an unpaired t test or a Mann‐Whitney U test.
Fig. S2. Immunophenotyping of the T‐cell subsets in the spleen of advanced maternal aged (AMA) dams. Number of (a) CD4+ T cells, (b) CD8+ T cells, (c) Th1 cells, (d) Th2 cells, (e) Th9 cells, (f) Th17 cells, (g) CD8+ cells expressing IFNγ, (h) CD8+ cells expressing IL‐4, (i) CD8+ cells expressing IL‐9, and (j) CD8+ cells expressing IL‐17A (n = 7‐10 each) in control and AMA dams. Mid‐lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min‐max range. The p values were determined by an unpaired t test or a Mann‐Whitney U test.
Fig. S3. Immunophenotyping of regulatory T cells (Tregs) in the uterine draining lymph nodes (ULN) and spleen of advanced maternal aged (AMA) and control dams. Number of (a) CD4+ Tregs and (b) CD8+CD25+FoxP3+ T cells in the ULN (n = 6‐10 each). Number of (c) CD4+ Tregs and (d) CD8+CD25+FoxP3+ T cells in the spleen (n = 7‐10 each). Mid‐lines indicate medians, boxes indicate interquartile ranges, and whiskers indicate min‐max range. The p values were determined by an unpaired t test or a Mann‐Whitney U test.
Table S1. Antibodies used for immunophenotyping.


