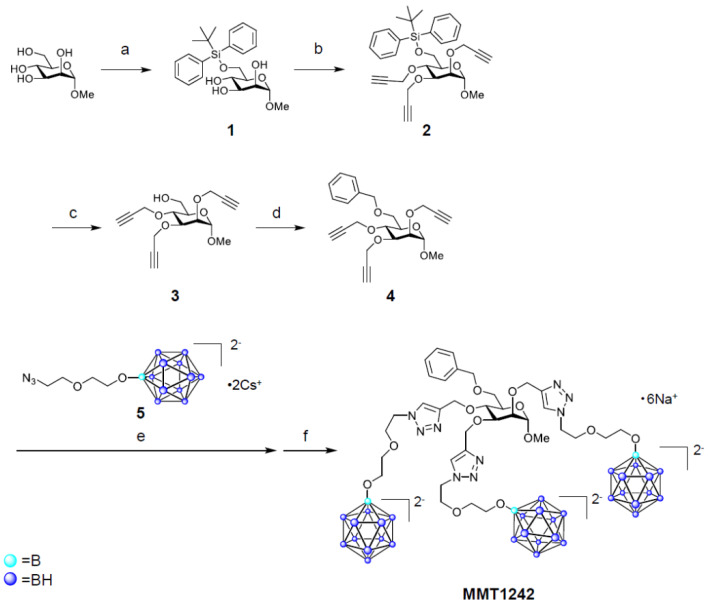
Figure 1.
Synthesis of MMT1242. Reaction conditions and yields: (a) tert-butylchlorodiphenylsilane, imidazole, N,N-dimethylformamide, 5 °C to rt (quant.); (b) sodium hydride, N,N-dimethylformamide, 5 °C to rt, then propargyl bromide, cat. tetrabutylammonium iodide, 5 °C to rt (68%); (c) tetrabutylammonium fluoride, tetrahydrofuran, 5 °C to rt (91%); (d) sodium hydride, N,N-dimethylformamide, 5 °C to rt, then benzyl bromide, rt (53%); (e) 5, copper (II) sulfate pentahydrate, sodium l-ascorbate, tert-butanol/H2O (1/2, v/v), 70 °C; (f) Amberlite® IR120B Na, H2O, (25%, 2 steps).