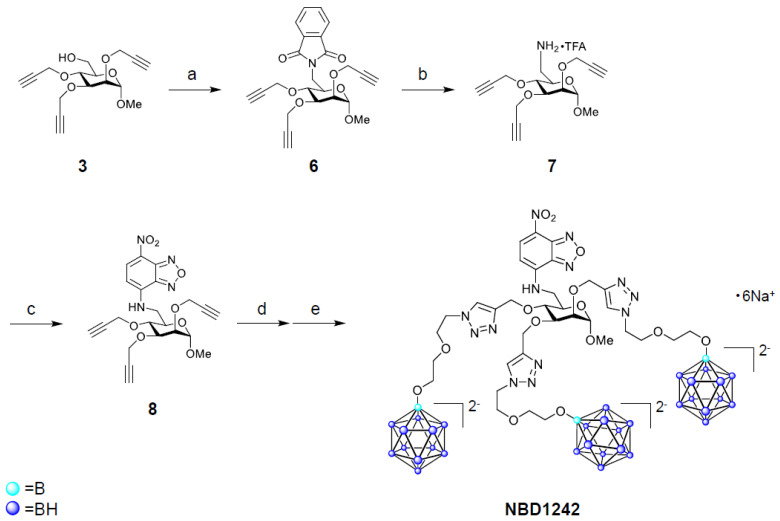
Figure 2.
Synthesis of NBD1242. Reaction conditions and yields: (a) phthalimide, triphenylphosphine, diethyl azodicarboxylate (40% in toluene), tetrahydrofuran, 5 °C to rt; (b) hydrazine monohydrate, ethanol, rt, then preparative RP-HPLC (containing 0.05% trifluoroacetic acid), (50%); (c) 4-fluoro-7-nitro-2,1,3-benzoxadiazole, triethylamine, N,N-dimethylformamide, 50 °C (38%); (d) 5, copper (II) sulfate pentahydrate, sodium l-ascorbate, tert-butanol/H2O (1/2, v/v), 70 °C; (e) H2O, Amberlite® IR120B Na (19%, 2 steps).