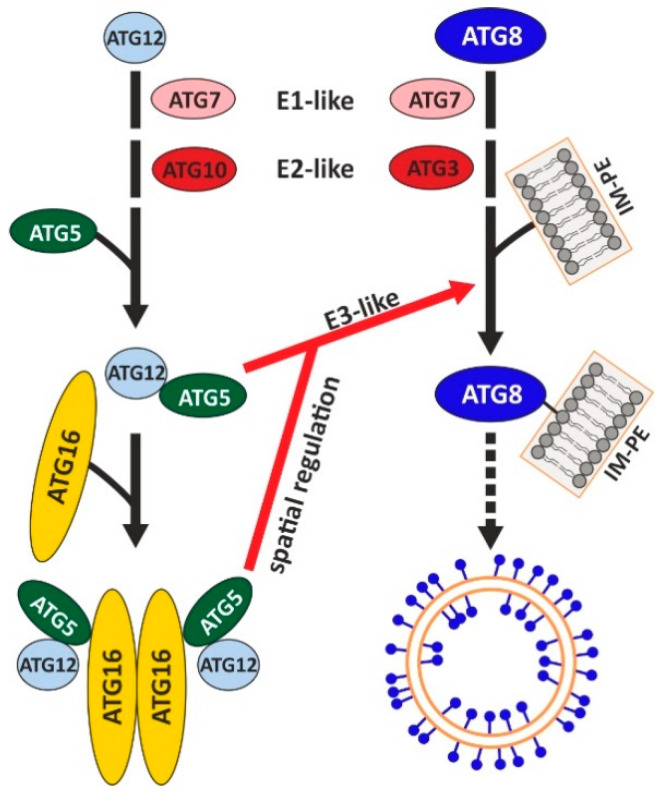
Figure 1.
Schematic depiction of the components and their interrelations of the two ubiquitin-like conjugation systems in autophagy. The ATG12 (left) and the ATG8 (LC3 in mammals) (right) conjugation systems are represented. Similar to protein ubiquitination, the ubiquitin-like protein ATG12 is activated by the E1 enzyme ATG7 and transferred to the E2 enzyme ATG10. Finally, ATG12 is covalently attached to its target protein ATG5 and two ATG12~5 conjugates in turn associate non-covalently with an ATG16 dimer and form a heterohexameric complex. Likewise, the ubiquitin-like protein ATG8 is also activated by ATG7, transferred to the E2 enzyme ATG3, and finally conjugated to PE via an amide bond. The ATG12~5/16 complex catalyses via its E3-like activity the conjugation of ATG8 to PE at the isolation membrane. The different components of the two conjugation systems are not drawn to scale. IM-PE, isolation membrane containing phosphatidylethanolamine. Modified from [39]. See text for further details.