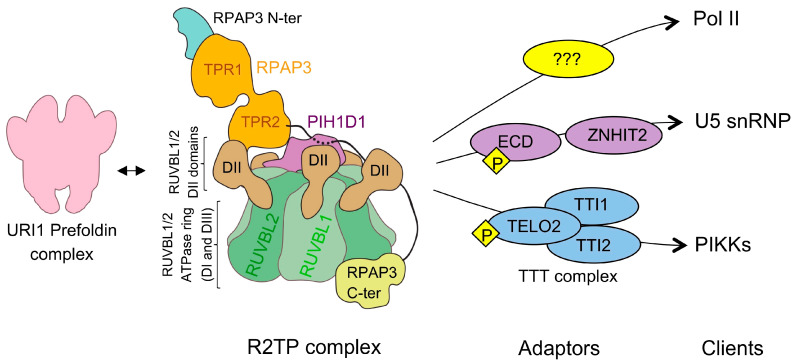
Figure 1.
The R2TP-based chaperone system. The cartoon represents the R2TP complex and its constituent proteins. The RUVBL1-RUVBL2 complex (labeled as RUVBL1/2 in the figure for simplicity) forms a hexameric ring that contains the ATPase domains and made of domains I (DI) and III (DIII). Domain II (DII) protrudes from each subunit and it can interact with other proteins. In the cartoon, the RUVBL1-RUVBL2 complex is seen from its side. The maturation of some clients requires the action of adaptors that have been proposed to link R2TP to its clients. Some adaptors contain a consensus motif recognized by PIH1D1 after CK2 phosphorylation, and in the figure, a “P” represents the phosphorylated version of this motif. The URI1 prefoldin complex associates with R2TP, and this interaction could be implicated in the assembly of Pol II. It is unknown if the functions of R2TP in the assembly of Pol II require specific adaptor proteins, and this is indicated as several question marks.