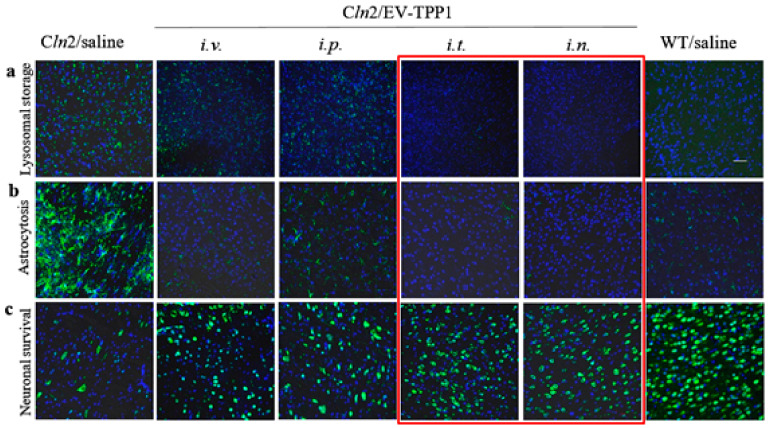
Figure 4.
Therapeutic effects of EV-TPP1 formulation administered via different routes in BD mouse model. CLN2 KO mice (1 month old, N = 6) were treated with EV-TPP1 through different routes two times a week for three weeks through i.v., or i.p. routes (4.5 × 1010 particles/ 150 µL/mouse; 15 mg/kg TPP1), or i.t. route (1.5 × 1010 particles/50 µL/mouse; 5 mg/kg TPP1), or i.n. route (0.6 × 1010 particles/20 µL/mouse; 2 mg/kg TPP1). BD mice and WT littermates injected with saline were used in control groups. Then, mice were sacrificed, perfused, brains were harvested, and slide were stained with: (a) Ab to lysosomal storage bodies (subunit C of mitochondrial ATP synthase); or (b) Ab to activated astrocytes (GFAP), or (c) Ab to neurons (NeuN), and secondary antibody goat anti-rabbit IgG H+L Alexa Flour 488 (green). Slides were washed and mounted with DAPI staining (blue). Treatments with EV-TPP1 via all examined routes resulted in reduced accumulation of lysosomal storage material in the brain (a) and neuroinflammation (b), as well as decreased neurodegeneration (c). Quantification of fluorescent levels (see Supplementary Table S1) indicates that i.t. and i.n. routes provided the most efficient therapeutic effects. The bar: 50 µm.