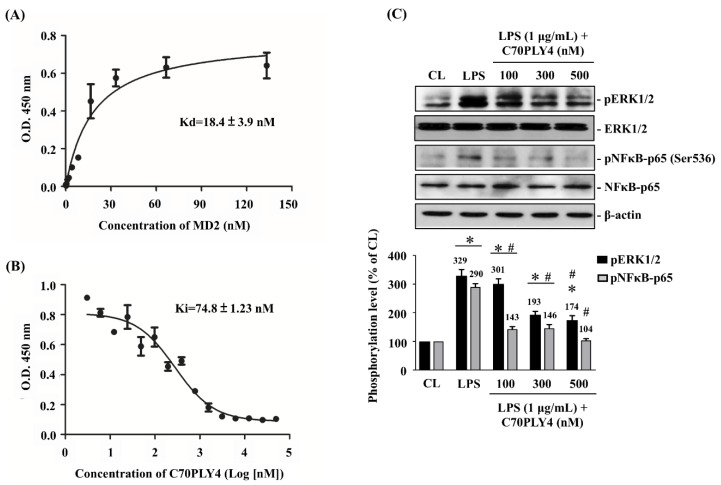
Figure 3.
C70PLY4 competes with MD2 for the binding site on TLR4 in vitro and inhibits LPS-induced ERK1/2 and NF-κB phosphorylation in HUVECs. (A) TLR4 (2 μg/mL) was coated onto a 96-well plate. MD2 was added to the wells at various concentrations (2-fold serial dilutions from 133.3 to 0.065 nM), and the Kd value was determined. (B) TLR4 (2 μg/mL) was coated onto a 96-well plate. Mixtures of MD2 (50 nM) and various concentrations (2-fold serial dilutions from 50 μM to 3.05 nM) of C70PLY4 were added to the wells, and the Ki value was determined. (C) The HUVECs were kept untreated as a control, treated with LPS (1 μg/mL) alone for 24 h, or co-treated with LPS (1 μg/mL) and C70PLY4 (100, 300, and 500 nM) for 24 h, and the protein phosphorylation of the ERK1/2 and NFκB-p65 subunit were examined. The results (C, upper panel) are representative of three independent experiments with similar results. The data for (A), (B) and (C, down panel) are the mean ± SEM from at least three independent experiments. *, p < 0.05 vs. control cells. #, p < 0.05 vs. LPS-only-treated cells.