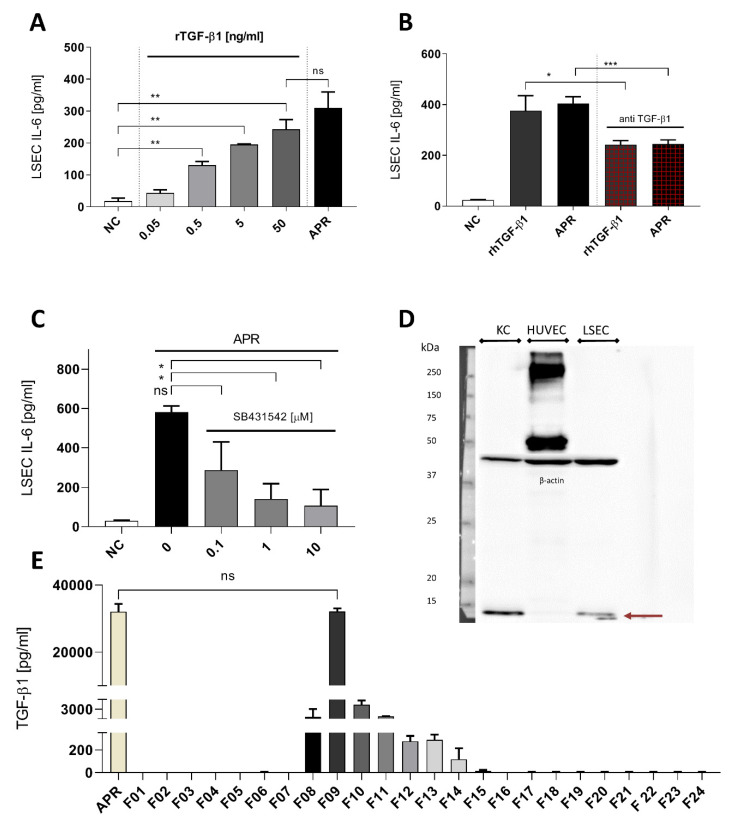
Figure 5.
Transforming growth factor β1 is a strong candidate to activated platelet releasate soluble factor. (A): LSEC titration with recombinant human TGF-β1 induced a dose-response on IL-6 secretion, and at 50 ng/mL, there was no statistically significant difference with human APR. NC: negative control, culture medium. (B): Using anti-human TGF-β1, we were able to reduce the effect of APR or recombinant human TGF-β1 by 50%. Antibody concentration: 5 μg/mL, rTGF-β1 concentration: 5 ng/mL. NC: negative control, tyrode buffer. (C): TGF-β1 signaling blockade using the serine–threonine inhibitor SB431542 at increasing concentration. NC: negative control, DMSO (using DMSO with APR did not result in a decrease of IL-6 production). (D): Western blot for expression of TGF-β1 in KC, HUVEC and LSEC. TGF-β1 appeared in reduced condition as a monomer of 12.8 kDa (burgundy arrow) and was detected in KC and LSEC but not in HUVEC. Loading was controlled with β-actin. 10% acrylamide gel. n = 1. (E): TGF-β1 concentration in each fraction, obtained by ELISA, directly correlated with the IL-6 response pattern by LSEC. Indeed, fraction 09 showed a dramatic amount of TGF-β1 that were similar to the amounts found in non-fractionated human APR, n = 2. If not otherwise specified, presented data are representative of 2 or 3 independent experiments. *** p-value between 0.0001 and 0.001, ** p-value between 0.001 and 0.01, * p-value between 0.01 and 0.05 (t-test), ns: non-significant.