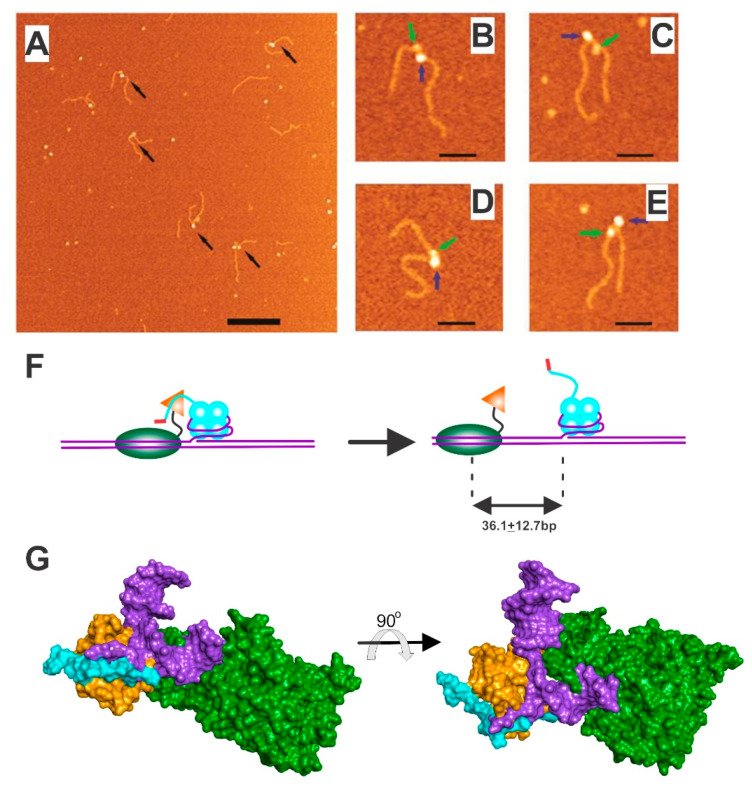
Figure 6.
SSB loads RecG and remodels the helicase in the process. (A) Large scale atomic force microscopy image of SSB-RecG-DNA complexes. Proteins are indicated by arrows. The scale bar is 200 nm. (B–E), Zoomed images of four double-blob complexes. Large (SSB) and small (RecG) blobs are indicated with blue and green arrows (scale bars 50 nm). (F). A model for RecG loading by SSB. SSB binds to the fork first and RecG via the linker from one monomer within the tetramer. Loading ensues, concomitant with the remodeling of the wedge domain (orange) so that only the helicase domains (green) can bind DNA. Once loaded, RecG slides, using thermal energy, on average, 36 bp ahead of the fork. (G). Molecular models of RecG bound to a fork (purple) and the SSB linker (cyan; a PXXP-containing ligand). The wedge domain is colored orange and helicase domains in green to match the schematic in panel F. As fork and linker binding are competitive, the cyan and purple colors overlap demonstrating that cannot occupy the same space. The two images are rotated 90° relative to each other to enable clear visualization. AFM images are from [43].