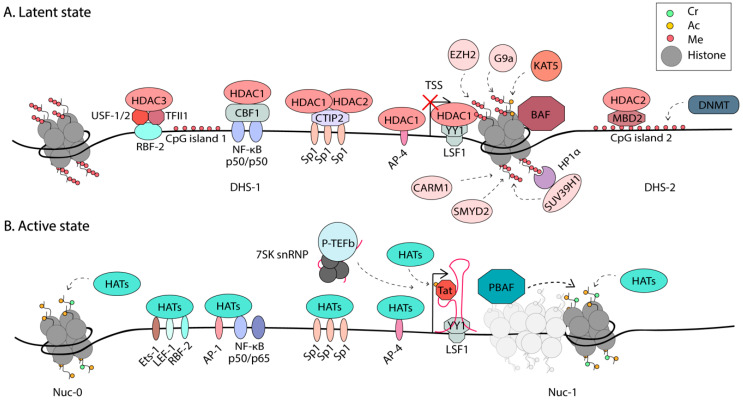
Figure 2.
Epigenetic regulation of HIV-1 transcription. This is a simplified overview of some of the factors involved in the modulation of HIV-1 latency. (A) Several proteins have been implicated in either the establishment or maintenance of HIV-1 latency. These include TFs, such as RBF2, NF-κB p50 homodimers, CTIP2, AP-4, and YY1, which directly bind to DNA and serve as docking sites or recruitment factors for other proteins, such as HDACs and HMTs. HDACs remove acetyl groups from the histone tails in nucleosomes. HMTs add methyl groups to histone tails. HMTs involved in HIV-1 latency include the well-established EZH2, G9a, SUV39H1, and the newly associated CARM1 and SMYD2. KAT5 has been recently implicated in histone 4 acetylation at the promoter, associated with HIV-1 latency. The “histone code,” or combination of PTMs (e.g., acetylation and methylation) at the promoter, promotes latency by increasing the affinity of nucleosomes for DNA, reducing DNA accessibility and through recruitment of repressive factors. This includes the SWI/SNF chromatin remodeling BAF complex, which positions Nuc-1 just downstream of the TSS and blocks transcriptional elongation. DNA methyltransferases (DNMTs) are thought to be recruited to the promoter and hypermethylate of the two CpG islands, leading to recruitment of HDACs via an interaction of MBD2 with the methyl groups. Finally, P-TEFb, required for the transition from transcriptional initiation to elongation, is held inactive through its association with the 7SK snRNP. (B) During activation, transcriptional repressors are replaced with activators, including NF-κB p50/p65 heterodimers and HATs, such as p300/CBP and hGCN5. HATs can acetylate or crotonylate histones, promoting a more open chromatin and recruiting the SWI/SNF chromatin remodeling PBAF complex. PBAF repositions Nuc-1 further downstream of the TSS, to allow transcription elongation. Furthermore, Tat is acetylated by p300 and binds to the secondary structure of the nascent TAR RNA, produced by the initiation of competent RNAPII. Tat can extract active P-TEFb either directly from free 7SK snRNP, or from 7SK snRNP tethered to the promoter, via an association with KAP1.