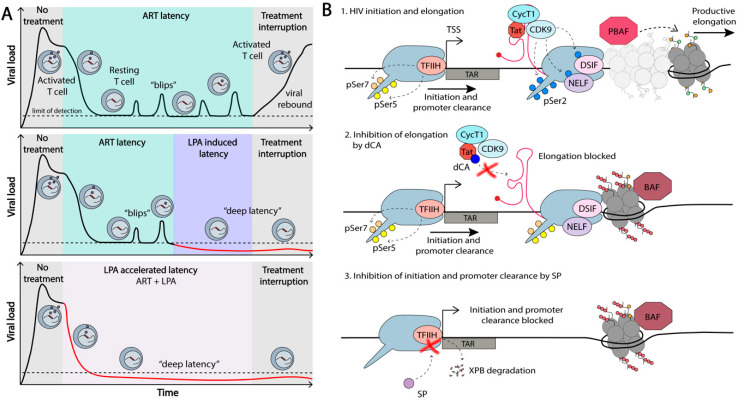
Figure 6.
Block-and-lock approach for a functional HIV-1 cure. (A) Top panel: Upon HIV-1 infection, within a few weeks, there is a peak of viral load (VL) circulating in the plasma of infected individuals. Upon ART initiation, the VL drops to below the limit of detection (50 viral copies/ml), termed “ART latency”. VL “blips,” or episodes of detectable viremia, and are observed in many individuals under ART. Upon discontinuation of ART, there is a rapid rebound of VL to pre-ART levels. Middle panel: The addition of a latency promoting agent (LPA), such as the Tat inhibitor dCA, to an ART regimen could suppress the ongoing transcriptional events, induce epigenetic silencing over time, and promote a state of “deep latency”. This might allow for safe ART discontinuation either without viral rebound, or a sufficiently low rebound, such that the immune system is able to control and suppress it. Bottom panel: The addition of an LPA at the initiation of ART might accelerate entry into latency, reduce transcriptional events, and potentially limit the size of the reservoir through a reduction in the total number of infected cells. The state of “deep latency” could be achieved earlier, reducing the amount of chronic immune activation in infected individuals and perhaps accelerating the epigenetic silencing of the provirus. (B) 1. When an HIV-1 infected CD4+T cell is activated, recruitment of the host TFs and RNAPII allows for efficient PIC formation, initiation of transcription, promoter escape, and some readthrough transcription to produce full length viral transcripts and, thus, Tat. A positive feedback loop is established, where Tat promotes productive elongation through recruitment of P-TEFb and the chromatin remodeler, PBAF. 2. The Tat inhibitor dCA binds to the basic domain of Tat and blocks Tat interaction with TAR RNA, blocking recruitment of P-TEFb directly to the HIV-1 promoter. Thus, transcriptional elongation is inhibited, and a more repressive chromatin environment can form at the promoter. 3. The small molecule SP causes degradation of the XPB subunit of TFIIH, which has been shown to inhibit transcription and reduce RNAPII occupancy at both the promoter as well as along the entire genome. SP provides another tool to explore the block-and-lock approach for a functional cure.