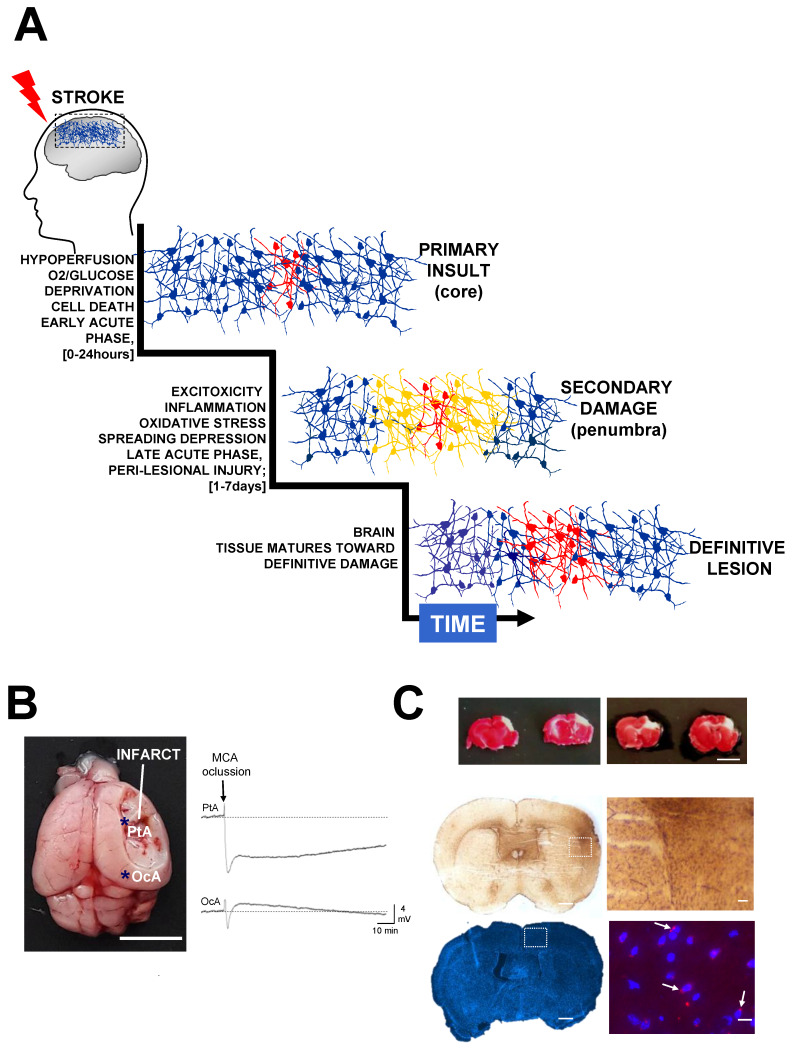
Figure 1.
Secondary injury increases lesion extension after stroke. (A) The sudden occlusion of a brain artery causes hypoperfusion and oxygen deprivation, producing a core of irreversibly damaged tissue surrounded by a penumbral area at risk of being damaged. After hypoxia, stroke triggers excitotoxicity, blood flow changes, inflammation, and oxidative stress, which produce secondary damage, thus extending the area of the lesion. The relevant time phases post-stroke have been defined as acute, sub-acute and chronic [30,31]. During the acute phase (hyperacute ~0–24 h; late acute ~1–7 days) it is possible to therapeutically neuroprotect the brain, thereby preventing the detrimental effects of secondary damage. The definitive damage is relatively well established several days after stroke, when neuroprotective agents are no longer efficient. During sub-acute (~7 days–6 months) and chronic (>6 months) phases, tissue reorganization can take place via rehabilitation and neural repair strategies. (B) Stroke produces peri-infarct depolarizations that cause vasoconstriction and blood flow reduction, propagating hypoperfusion to peri-lesional areas and causing additional damage. The image shows a mouse brain (MCAO model, scale bar 0.5 cm) 24 h after permanent ischemia (MCA ligation). Immediately after MCA occlusion, a wave of terminal depolarization was electrophysiological recorded in peri-lesional areas (parietal cortex, PtA) bordering the infarct core (asterisk in the image). Note the existence of brief depolarization waves in non-damaged distant areas (occipital, OcA), which, in metabolically compromised ischemic tissues, also might cause intense vasoconstriction and hypoperfusion. (C) At the top, representative coronal brain sections from two mice stained with TTC (2,3,5-triphenyltetrazolium chloride) 24 h after MCA occlusion at distal level respect the Circle of Willis. In this specific stroke model, the infarct area (in a white colour) is mainly restricted to the cortex (scale bar 0.5 cm) [83,84,85]. In the middle, as part of the inflammatory response, an intense astrogliosis (Glial Fibrillary Acidic Protein staining) can usually be detected in the infarcted hemisphere in relation to the contralateral hemisphere (scale bars 700 μm and 100 μm, respectively). In the bottom, representative brain sections stained with dihydroethidium (DHE) to detect reactive oxygen species and intracellular superoxide. In this example, as early as 8 h after MCAO, the most intense fluorescence was detected in the peri-lesional tissue in perinuclear locations (the nuclei are stained with DAPI, scale bars 700 μm and 10 μm respectively).