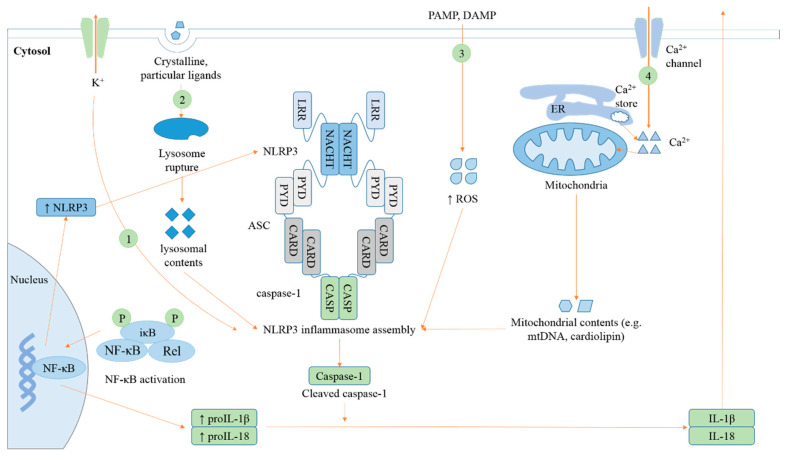
Figure 1.
Models for nucleotide-binding domain, leucine-rich-repeat-containing family, pyrin domain-containing 3 (NLRP3) inflammasome activation. Four models for NLRP3 inflammasome activation that may not be exclusive have been proposed: (1) Multiple signal transduction pathways triggered by pathogen-associated molecular patterns (PAMPs)(/danger-associated molecular patterns DAMPs) converge on K+ efflux [20,21,22], leading to NLRP3–NEK (NIMA related kinase) interaction and NLRP3 inflammasome activation [23,24]. (2) Engulfment of crystalline or particular ligands, including monosodium urate (MSU) [25], silica [26], and amyloid-β [27], leads to lysosomal damage and resultant cytosolic release of lysosomal contents, which activates the NLRP3 inflammasome. (3) NLRP3 agonists, including ATP [28], MSU, and asbestos [29] trigger production of reactive oxygen species (ROS). This common pathway engages the NLRP3 inflammasome. However, later studies demonstrated that ROS only control NLRP3 inflammasome activation in the priming step, but not in the activation step [30]. ROS generation is even dispensable for both the priming and activation in certain circumstances [20]. (4) Ca2+ mobilization from extracellular milieu or endoplasmic reticulum (ER) Ca2+ stores induced by NLRP3 agonists leads to elevation of cytosolic Ca2+ concentration. Excessive and/or sustained mitochondrial Ca2+ influx results in Ca2+ overload, mitochondrial damage, and release of mitochondrial contents, triggering NLRP3 inflammasome activation [31,32,33].