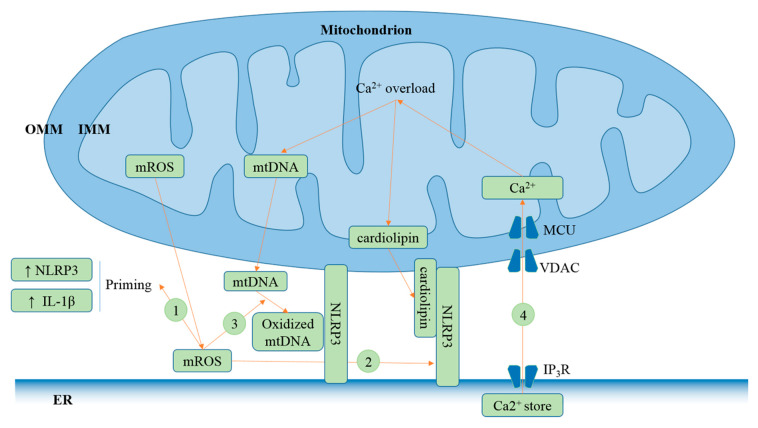
Figure 3.
MAMs contribute to NLRP3 inflammasome activation. (1) ER–mitochondria proximity plays a crucial role in the signaling pathways associated with short-lived ROS. Its generation induces increased expression of NLRP3 and substrate of the inflammasome IL-1β [30]. NLRP3 level is critical for the priming step. The inflammasome can be activated by ATP alone without lipopolysaccharide (LPS) priming in macrophages overexpressing NLRP3 [74]. (2) mROS promote NLRP3 association with mitochondria [73], which subsequently induces its interaction with cardiolipin that translocates from the inner mitochondrial membrane (IMM) to the outer mitochondrial membrane (OMM) in response to the NLRP3 stimuli [72]. (3) mROS mediate oxidation of mtDNA [75]. Oxidized mtDNA binds to NLRP3, triggering NLRP3 inflammasome activation [7]. (4) The close proximity between the ER and mitochondria facilitates rapid Ca2+ mobilization to mitochondria from the ER through mitochondrial calcium uniporter (MCU) and voltage-dependent anion-selective channel (VDAC), leading to mitochondrial Ca2+ overload, mitochondrial dysfunction, release of mtDNA and cardiolipin externalization [31,33,76].