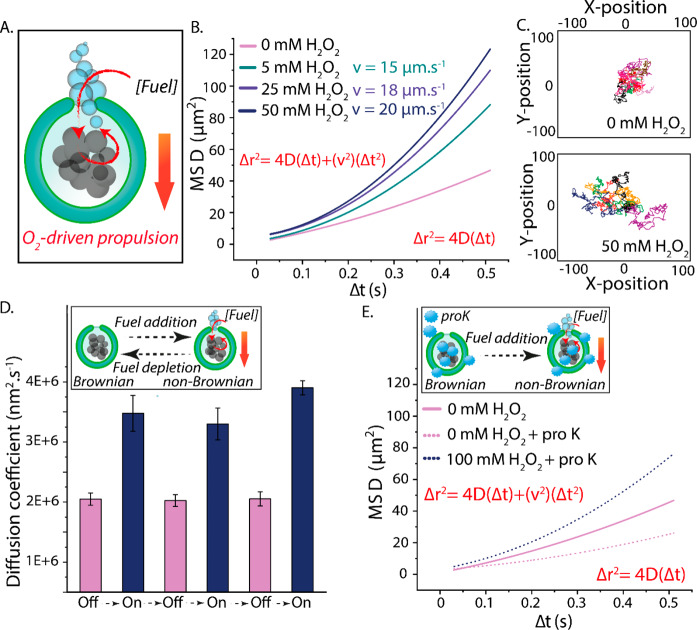
Figure 2.
(A) Schematic demonstrating the spatial positioning of catalytic MnPs within stomatocytes, MnPs’ capacity to convert a range of fuel concentrations into O2 nanobubbles, and the (hypothesized) mechanism by which motion occurs. (B) MSD and velocity of hybrid nanomotors in the presence of a range of H2O2 concentrations. The velocities were theoretically calculated from MSD = (4D)Δt + (v2)(Δt2). NTA measurements were performed by diluting MnP-loaded stomatocytes (1:1000) in 1× PBS ([polymer] = 5 μg mL–1). Thereafter, aliquots of H2O2 were added (to reach a final concentration of 5, 25, or 50 mM). (C) Tracked Brownian and non-Brownian trajectories in the absence and presence of fuel, respectively. (D) Diffusion coefficients of MnP-loaded stomatocytes as a result of multiple cycles of fuel addition and depletion. In order to ensure complete depletion of fuel, the time interval between experiments (fuel addition) was 1 day. (E) MSDs of hybrid stomatocytes exposed to 50 μg mL–1 proteinase K (dotted line).