Fig. 1.
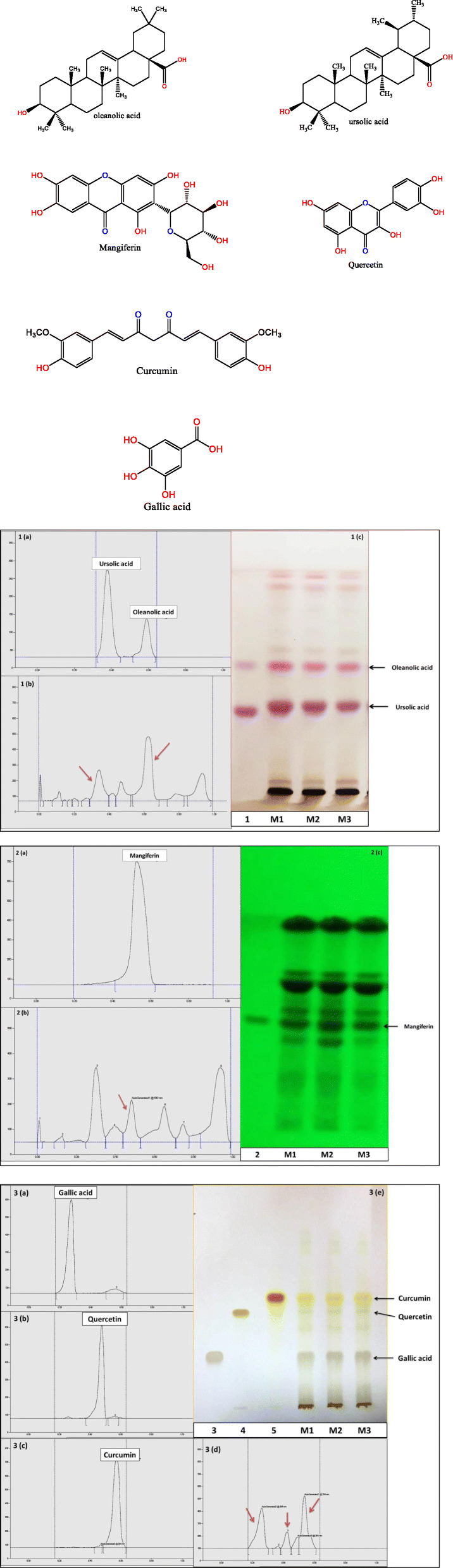
Densitogram obtained from Standard- ursolic and oleanolic acid (1 a) using n-hexane: ethyl acetate: acetone (16.4: 3.6: 0.2 v/v) mobile phase; formulation samples containing ursolic and oleanolic acid (1 b); Standard-mangiferin (2 a); formulation samples containing mangiferin (2 b) using ethyl acetate: glacial acetic acid: formic acid: water (20: 2.2: 2.2: 5.2 v/v) mobile phase; Standard- gallic acid (3 a); Standard- quercetin (3 b); Standard- curcumin (3 c) using toluene: ethyl acetate: formic acid (13.5: 9: 0.6 v/v) mobile phase; and formulation samples contains gallic acid, quercetin and curcumin (3 d). HPTLC fingerprinting (1c; 2c; 3e) showing track 1; 2; 3–5: STANDARD-oleanolic and ursolic acid; mangiferin; gallic acid; quercetin and curcumin respectively where tracks M1, M2 and M3 signifying marker compounds in three different commercial formulations (MC1, MC2 and MC3)
