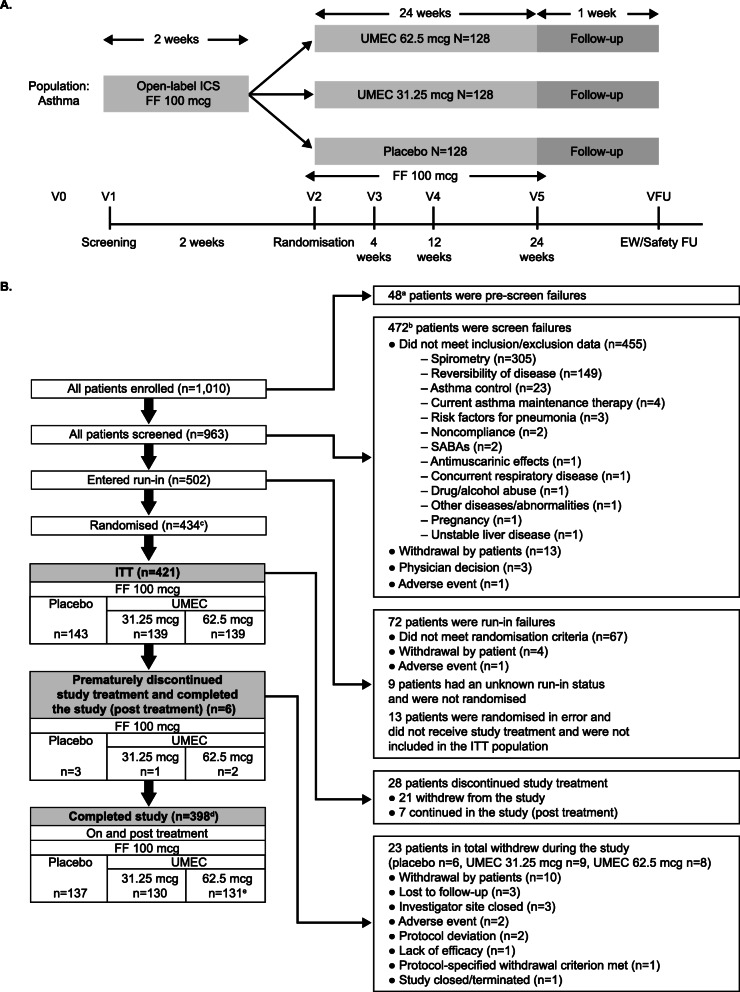
Fig. 1.
(a) Study design and (b) patient disposition. aOne patient failed pre-screening and entered screening, and was counted as both a pre-screen failure and in the all patients screened population. bEleven patients failed screening and entered the run-in period, and were counted as both screen failures and in the entered run-in population. cThe study planned to randomise 384 patients. dOne patient had an unknown study completion status. ePatient 954 in the UMEC 62.5 mcg group discontinued study treatment the day prior to Visit 5 (Week 24) and was not dosed at that clinic visit. However, this patient was not reported as prematurely discontinuing study treatment in the eCRF and was counted in the completed study population. On treatment was defined as study treatment start date day (inclusive) to study treatment stop date + 1 (inclusive). Post treatment was defined as study treatment stop date + 1 day (exclusive) to Visit 5/EW Visit date (as applicable) (inclusive). The all patients enrolled population included all patients for whom a record exists in the database. The all patients screened population included all patients who completed ≥1 screening procedure. The randomised population included all patients who were randomised. The ITT population included all patients who were randomised, excluding those who were randomised in error and did not receive study treatment. AE, adverse event; eCRF, electronic Case Report Form; EW, early withdrawal; FF, fluticasone furoate; FU, follow-up; ICS, inhaled corticosteroid; ITT, intent-to-treat, SABA, short-acting β2-agonist; UMEC, umeclidinium; V, visit