Fig. 1. Basic TIS method overview.
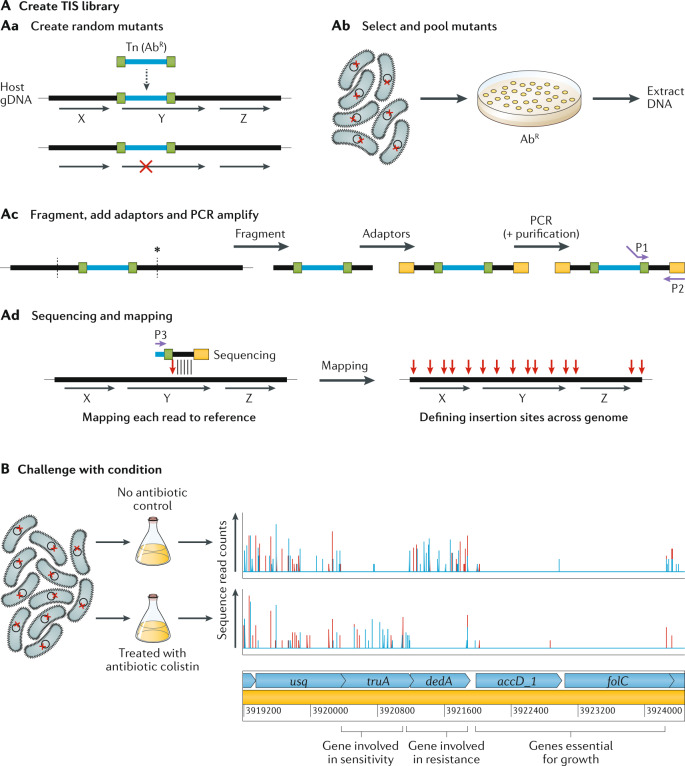
A | Creation of the transposon-insertion sequencing (TIS) library has four steps. The first is to create random transposon (Tn) mutants (part Aa). The horizontal black lines and arrows represent the host’s genomic DNA (gDNA) and the coding regions of genes are marked ‘X’, ‘Y’ and ‘Z’. The horizontal blue line is the transposon containing an antibiotic resistance (AbR) selection marker and bounded by inverted repeats, shown in green. When the transposon inserts itself into the gDNA, the disruption in a gene (gene Y in this example) is shown by a red cross. The second step is to select and pool mutants (part Ab). The red cross represents a single mutation in each cell; these cells are selected, for instance on antibiotic-containing agar plates, and pooled and DNA is extracted. The third step is fragmentation, addition of adaptors and PCR amplification (part Ac). Fragmentation (vertical dashed lines) can be enzymatic or by shearing (depending on the version of TIS). Sequencing adaptors (yellow rectangles) are then added, and primers (purple arrows) P1 and P2 are used for PCR amplification. Step 4 is sequencing and mapping (part Ad). Sequences out from the transposon end (using primer P3) are mapped onto the reference genome and the transposon insertion point is determined (vertical red arrow) and mapped for each mutant. Genes that cannot tolerate insertions (gene Z in this example) will not have any TIS reads mapped. B | Challenging the TIS library — in this example with an antibiotic, colistin (bottom row), compared with an untreated control (top row) (data from ref.42). The vertical lines denote the density of insertions at each insertion site, and red and blue denote forward or reverse insertion direction, respectively. Below are the predicted genes, in light blue. The first gene (usq) has equivalent numbers of insertions in the treated and untreated samples and thus has no effect on fitness in colistin. The next gene (truA) has relatively more insertions in the treated sample compared with the control (its mutants have increased fitness in colistin) and thus is considered a sensitivity gene. The next gene (dedA) is an experimentally confirmed resistance gene42 and it has decreased insertions in the treated sample (mutants have decreased fitness in colistin). The last two genes (accD_1 and folC) have no insertions in either the treated sample or the untreated sample and are thus considered essential for growth.