Abstract
Introduction
We herein present a case of malignant melanoma of the male urethra with an increased serum 5‐S‐cysteinyldopa concentration.
Case presentation
A 77‐year‐old man visited our hospital complaining dysuria and a dark brown mass protruding from the external urethral meatus. His serum 5‐S‐cysteinyldopa concentration was elevated beyond the upper limit of the reference range. Biopsy of the tumor was performed, and the histological diagnosis was malignant melanoma. He underwent total penectomy, and the serum 5‐S‐cysteinyldopa concentration was normalized. He remained alive without evidence of locoregional recurrence or distant metastases for 6 months after surgery.
Conclusion
Malignant melanoma of the male urethra is uncommon. The prognosis is favorable if it is detected in its early stages. This case report suggests that measurement of the serum concentration of 5‐S‐cysteinyldopa, a melanin metabolite, is useful for early diagnosis of male urethral melanoma.
Keywords: 5‐S‐cysteinyldopa, male, malignant melanoma, penis, urethra
Abbreviations & Acronyms
- 5‐S‐CD
5‐S‐cysteinyldopa
- SLNB
sentinel lymph node biopsy
Keynote message.
Malignant melanoma of the male urethra is extremely rare, and most cases are diagnosed at an advanced stage. Approximately half of the affected patients have lymph node metastasis at the time of diagnosis, which carries a very poor prognosis. In this case report, we describe the diagnosis and treatment of malignant melanoma of the male urethra with an increased serum 5‐S‐CD concentration and discuss the use of 5‐S‐CD as a useful tumor marker.
Introduction
The most common sites of primary malignant melanoma are the skin and eye. Malignant melanoma of the genitourinary tract is extremely rare, representing <1% of all melanomas.1, 2 Melanoma of the penis is <2% of all primarily penile malignant lesions.3 Malignant melanoma of the penis may be located on the glans penis, prepuce, penile shaft, or urethral meatus.4 Although early diagnosis is important for proper treatment and improved survival rates of malignant melanoma of the penis, most cases are found in an advanced state, and almost 50% of affected patients have inguinal lymph node metastasis at the time of diagnosis.5 Here we report a case of a 77‐year‐old man with melanoma of the distal urethra who was treated by total penectomy. 5‐S‐CD, which has been suggested as a diagnostic marker of malignant melanoma, was elevated and returned to the normal range after the operation.
Case presentation
A 77‐year‐old man was referred to our urology outpatient clinic for evaluation of difficult urination and a mass protruding from the external urethral meatus. He had no family history of malignant disease, including melanoma. Physical examination revealed a 5‐mm dark brown mass projecting from the external urethral meatus. Melanin pigmentation of the glans penis was also noted (Fig. 1a). No other skin lesions suspicious of malignant melanoma were observed. On cystourethroscopy, we found an oval mass located on the dorsal distal urethra accompanied by surrounding dark brown lesions extending about 6 cm from the external urethral meatus (Fig. 1b). The proximal urethra and the bladder were normal. Biopsy of the mass lesion established the diagnosis of malignant melanoma, which was consistent with the patient's elevated serum 5‐S‐CD concentration (16.3 nmol/mL; reference range 1.5–8.0 nmol/mL). Computed tomography and magnetic resonance imaging revealed no findings of metastasis. The patient underwent total penectomy without pelvic lymph node dissection. Gross examination of the resected penis revealed a 5‐mm mass and a 6‐cm area of melanin pigmentation in the pendulous urethra (Fig. 1c). On histopathological examination, the tumor thickness was 2.1 mm without ulceration, and the resection margin was 22 mm. On immunohistochemical examination, tumor cells were positive for HMB‐45, SOX10, and melanin A (Fig. 2). Based on these findings, the patient was diagnosed as melanoma of the urethra (American Joint Committee on Cancer stage IIA; pT3aN0M0). After surgery, the serum 5‐S‐CD concentration gradually decreased and returned to the reference range at 6 months. The patient has been alive without signs of recurrence for 6 months of follow‐up.
Figure 1.
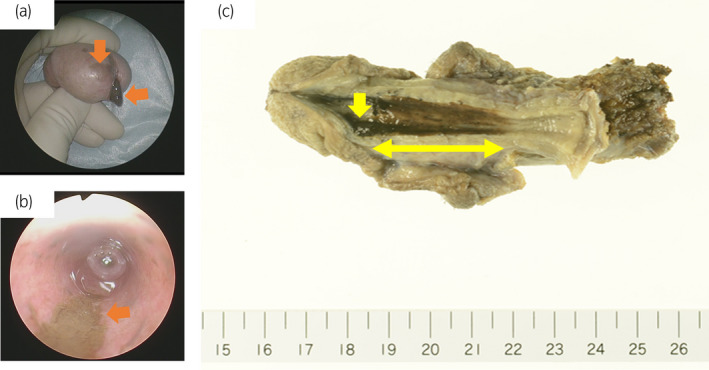
Malignant melanoma of both the glans penis and urethra. (a) A 5‐mm dark brown mass was projecting from the external urethral meatus, and the glans penis exhibited melanin pigmentation. (b) Cystourethroscopy revealed an oval mass located on the dorsal distal urethra accompanied by surrounding dark brown lesions extending about 6 cm from the external urethral meatus. (c) Longitudinal incision of the penis showed a 5‐mm mass and a 6‐cm area of melanin pigmentation in the pendulous urethra.
Figure 2.
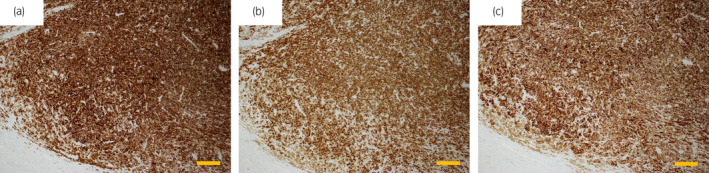
Immunohistochemistry findings. The tumor cells were positive for (a) HMB‐45, (b) SOX10, and (c) melanin A. Scale bar shows 100 μm.
Discussion
Malignant melanoma of the male urethra and penis is rare.6 This type of melanoma occurs in men aged 50–70 years, which is older than the peak incidence of skin melanoma (40–49 years).3, 7, 8 Melanoma of urethral origin is frequently misdiagnosed clinically because the symptoms of urethral melanoma is very similar to more common urothelial malignancies.9 This misdiagnosis leads to delay in treatment. Microscopic, immunohistochemical, and ultrastructural examination may play an important role in establishing the diagnosis in many cases.10
Early detection of the disease and early diagnosis of recurrence could improve treatment results and survival. Several serological tumor markers for melanoma have been reported, including various cytokines, cytokine receptors, cell adhesion molecules, S100 protein, melanoma inhibitory activity, neuron‐specific enolase, lipid‐bound sialic acid, and melanin metabolites.11 The serum levels of these markers increase in the advanced stages of disease. Although these markers are useful for detecting disease progression or predicting the therapeutic outcome, they are not useful for detection of melanoma at an early stage. 5‐S‐CD, a melanin metabolite, is produced in melanocytes and melanoma cells during biosynthesis of melanin.12 It is detectable in urine and serum. Several studies have shown that the serum 5‐S‐CD concentration is the most useful marker of disease progression.13, 14, 15 According to a report by Peterson et al.,16 the serum 5‐S‐CD concentration is useful not only for patient follow‐up but for identifying melanoma at an early stage. In the current case, the serum 5‐S‐CD concentration was already increased at the first visit, then gradually decreased and finally normalized after surgery. 5‐S‐CD is produced not only in patients with malignant melanoma but also in healthy individuals. The serum 5‐S‐CD concentration is also influenced by race and age, and there are also diurnal and seasonal variations. From these facts, it is considered that there is individual difference in the reduction rate of serum 5‐S‐CD concentration after surgery.
Treatment options for melanoma of the penis and male urethra are circumcision, urethrectomy, and total or partial penectomy.17 In cases of penile melanoma, circumcision is recommended for lesions of the foreskin alone, partial penectomy for lesions of the glans alone, and total penectomy for lesions of the glans and shaft. In cases of penile urethral melanoma, urethrectomy is recommended for masses with a depth of ≤2 mm, and penectomy, urethrectomy, or perineal urethrectomy is recommended for masses with the depth of >2 mm.17 The necessary surgical margin for skin melanoma based on the 1992 National Institution of Health Consensus Conference on Melanoma is 0.5 cm for Tis disease, 1 cm for tumors with a depth of <1 cm, 2 cm for tumors with a depth of <1–2 cm, and 2–4 cm for tumors with a depth of <4 cm.18 In the present case, the patient underwent total penectomy according to the appropriate surgical margin described above. The primary treatment of melanoma of the penis and urethra is surgical, although there is no consensus regarding the extent of treatment, such as inguinal lymph node dissection and pelvic lymph node dissection. Elective lymph node dissection is not recommended in patients with malignant melanoma of the male urethra and penis due to the low possibility of positive findings, and lack of benefit on the overall survival.19 In the present case, we did not perform lymph node dissection because there was no apparent inguinal lymph node swelling on computed tomography. However, SLNB is considered to be useful in the evaluation of melanomas with a thickness of ≥1 mm according to the National Comprehensive Cancer Network guidelines. Therefore, SLNB of inguinal lymph node dissection might be considered in our case.
Our patient was classified as having Stage IIA disease, which has a favorable prognosis (a 5‐year survival rate of 79%), if treated appropriately.20 Therefore, the patient did not receive adjuvant chemotherapy after surgery. However, the prognosis for patients with lymph node metastasis is extremely poor, and many patients die of the disease despite appropriate surgery and multi‐agent chemotherapy. Thus, detection of the disease at an early stage is important. However, immune checkpoint inhibitors such as nivolumab and ipilimumab have recently been approved for the treatment of advanced melanoma or adjuvant therapy after surgery. In cases of advanced malignant melanoma of the penis and male urethra, these treatment modalities may also improve the patients’ prognosis.
In conclusion, malignant melanoma might be curable if the disease is diagnosed at an early stage with favorable pathological characteristics. Measurement of the serum 5‐S‐CD concentration might be helpful for early detection of the disease.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgment
We thank Angela Morben, DVM, ELS from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
References
- 1. Gupta R, Bhatti SS, Dinda AK, Singh MK. Primary melanoma of the urethra: a rare neoplasm of the urinary tract. Int. Urol. Nephrol. 2007; 39: 833–6. [DOI] [PubMed] [Google Scholar]
- 2. Das P, Kumar N, Ahuja A et al Primary malignant melanoma at unusual sites: an institutional experience with review of literature. Melanoma Res. 2010; 20: 233–9. [DOI] [PubMed] [Google Scholar]
- 3. Stillwell TJ, Zincke H, Gaffey TA, Woods JE. Malignant melanoma of the penis. J. Urol. 1988; 140: 72–5. [DOI] [PubMed] [Google Scholar]
- 4. Tallerman A. Malignant melanoma of the penis. Urol. Int. 1972; 27: 66–80. [DOI] [PubMed] [Google Scholar]
- 5. Rogers RS 3rd, Gibson LE. Mucosal, genital, and unusual clinical variants of melanoma. Mayo Clin. Proc. 1997; 72: 362–6. [DOI] [PubMed] [Google Scholar]
- 6. Demitsu T, Nagato H, Nishimaki K et al Melanoma in situ of the penis. J. Am. Acad. Dermatol. 2000; 42: 386–8. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Yuan H, Wang A, Zhang Z, Wu J, Wei Q. Malignant melanoma of the penis and urethra: one case report. World J. Surg. Oncol. 2014; 12: 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Geel AN, den Bakker MA, Kirkels W et al Prognosis of primary mucosal penile melanoma: a series of 19 Dutch patients and 47 patients from the literature. Urology 2007; 70: 143–7. [DOI] [PubMed] [Google Scholar]
- 9. Calcagno L, Casarico A, Bandelloni R, Gambini C. Primary malignant melanoma of male urethra. Urology 1991; 37: 366–8. [DOI] [PubMed] [Google Scholar]
- 10. Oliva E, Quinn TR, Amin MB et al Primary malignant melanoma of the urethra: a clinicopathologic analysis of 15 cases. Am. J. Surg. Pathol. 2000; 24: 785–96. [DOI] [PubMed] [Google Scholar]
- 11. Brochez L, Naeyaert JM. Serological markers for melanoma. Br. J. Dermatol. 2000; 143: 256–68. [DOI] [PubMed] [Google Scholar]
- 12. Benathan M. Modulation of 5‐S‐cysteinyldopa formation by tyrosinase activity and intracellular thiols in human melanoma cells. Melanoma Res. 1996; 6: 183–9. [DOI] [PubMed] [Google Scholar]
- 13. Hasegawa M, Takata M, Hatta N, Wakamatsu K, Ito S, Takehara K. Simultaneous measurement of serum 5‐S‐cysteinyldopa, circulating intercellular adhesion molecule‐1 and soluble interleukin‐2 receptor levels in Japanese patients with malignant melanoma. Melanoma Res. 1997; 7: 243–51. [DOI] [PubMed] [Google Scholar]
- 14. Hirai S, Kageshita T, Kimura T et al Serum levels of sICAM‐1 and 5‐S‐cysteinyldopa as markers of melanoma progression. Melanoma Res. 1997; 7: 58–62. [DOI] [PubMed] [Google Scholar]
- 15. Meyerhoffer S, Lindberg Z, Hager A, Kagedal B, Rosdahl I. Urinary excretion of 5‐S‐cysteinyldopa and 6‐hydroxy‐5‐methoxyindole‐2‐carboxylic acid in children. Acta Derm. Venereol. 1998; 78: 31–5. [DOI] [PubMed] [Google Scholar]
- 16. Peterson LL, Woodward WR, Fletcher WS, Palmquist M, Tucker MA, Ilias A. Plasma 5‐S‐cysteinyldopa differentiates patients with primary and metastatic melanoma from patients with dysplastic nevus syndrome and normal subjects. J. Am. Acad. Dermatol. 1988; 19: 509–15. [DOI] [PubMed] [Google Scholar]
- 17. Sanchez‐Ortiz R, Huang SF, Tamboli P, Prieto VG, Hester G, Pettaway CA. Melanoma of the penis, scrotum and male urethra: a 40‐year single institution experience. J. Urol. 2005; 173: 1958–65. [DOI] [PubMed] [Google Scholar]
- 18. Bevan‐Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the M.D. Anderson Cancer Center Experience. J. Urol. 2002; 167: 1638–42. [PubMed] [Google Scholar]
- 19. Cascinelli N, Morabito A, Santinami M, MacKie RM, Belli F. Immediate or delayed dissection of regional nodes in patients with melanoma of the trunk: a randomised trial. WHO melanoma programme. Lancet 1998; 351: 793–6. [DOI] [PubMed] [Google Scholar]
- 20. Balch CM, Gershenwald JE, Soong SJ et al Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009; 27: 6199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]


