Abstract
Introduction
Only few cases of renal dysfunction in patients with situs inversus totalis have been reported. Thus, studies on kidney transplantation in patients with this condition are still limited.
Case presentation
We present three cases of end‐stage renal disease patients with situs inversus totalis: a 30‐year‐old man, 21‐year‐old woman, and 31‐year‐old man. Each left living‐donor kidney was transplanted in the right iliac fossa in the usual way. Because of the anatomical reversal and right external iliac vein being deep, the internal iliac vein was cut for venous anastomosis in one patient. Another one patient developed temporary congestive kidney, which was speculated to be due to poor blood flow in the renal vein. All recipients could be weaned off dialysis, with stable allograft function.
Conclusion
Kidney transplantation in patients with situs inversus totalis is the same as anatomical normal cases, except that attention is paid to venous anastomosis.
Keywords: end‐stage renal disease, kidney transplantation, living‐donor, situs inversus, venous anastomosis
Abbreviations & Acronyms
- Anti‐HLA DSA
donor‐specific anti‐human leukocyte antigen antibody
- CGN
chronic glomerular nephropathy
- CT
computed tomography
- ESRD
end‐stage renal disease
- FSGS
focal segmental glomerulosclerosis
- HLA
human leukocyte antigen
- KT
kidney transplantation
- MRI
magnetic resonance imaging
- SI
situs inversus
Keynote message.
Although studies on KT in an ESRD patient with SI totalis have not been well reported, it is the same as anatomical normal cases except that attention is paid to venous anastomosis.
Introduction
SI is a rare congenital anomaly in which abdominal and thoracic organs and blood vessels are totally or partially reversed from their normal positions. SI totalis occurs in one in 20 000 live births.1 It is often associated with congenital multiple organ anomalies; dysplasia2 and hypoplasia3 were previously reported in the kidney. Although nephrotic syndrome4, 5, 6 and renal amyloidosis7, 8 have been reported in SI patients, the mechanisms of occurrences remain unknown owing to scarcity of cases. To our knowledge, KT surgery and postoperative course in an ESRD patient with SI totalis have not been previously reported. Herein, we present three cases of ESRD patients with SI totalis who successfully underwent living‐donor KT.
Case presentation
Case 1
A 30‐year‐old man who underwent kidney biopsy more than 10 years ago at another hospital was diagnosed with ESRD due to a suspicion of FSGS. Immunological evaluation showed negative crossmatch test results, and there were no anti‐HLA DSAs. As immunosuppression induction, tacrolimus, mycophenolate mofetil, methylprednisolone, and basiliximab were started. He also received preoperative plasma exchange therapy and rituximab to prevent recurrence of FSGS. ABO‐compatible KT was performed from his healthy 57‐year‐old father.
Case 2
A 21‐year‐old woman who was diagnosed with ESRD due to CGN without kidney biopsy underwent hemodialysis for 5 months. She had hypertension prior to hemodialysis. Immunological evaluation showed negative crossmatch test results, and there were no anti‐HLA DSAs. Immunosuppression was induced with the same regimen as in case 1. She received ABO‐compatible KT from her healthy 58‐year‐old mother. The recipients of cases 1 and 2 were siblings, and their donors were their parents.
Case 3
A 31‐year‐old man who was diagnosed with ESRD due to CGN without kidney biopsy underwent hemodialysis for 6 months. He underwent surgery for bilateral vesicoureteral reflux 15 years ago, but there was no recurrence. He had hyperuricemia prior to hemodialysis. Immunological evaluation showed negative crossmatch test results, and there were no anti‐HLA DSAs. Immunosuppression was induced with the same regimen as above, and he received ABO‐compatible KT from his healthy 61‐year‐old father.
Patient characteristics are shown in Table 1. CT showed mirror‐image location of the organs and blood vessels with bilateral atrophic kidneys in all cases (Fig. 1). Surgical and postoperative outcomes of KT are shown in Table 2. The left kidney was procured using pure retroperitoneoscopic donor nephrectomy in all cases. In the same way as usual, each left donor kidney was transplanted at the right iliac fossa of the recipient via a Gibson incision and retroperitoneal approach. The renal artery was anastomosed end‐to‐end to the internal iliac artery or end‐to‐side to the external iliac artery, and the renal vein was anastomosed end‐to‐side to the external iliac vein (Fig. 2). The external iliac vein in case 3 was deep for the anastomosis procedure; thus, the internal iliac vein was cut. There were no perioperative complications, and all patients entered the intensive care unit, with urine discharge.
Table 1.
Characteristics of patients
| Variables | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age (years) | 30 | 21 | 31 |
| Sex | Man | Woman | Man |
| Body mass index (kg/m2) | 21.9 | 19.7 | 27.5 |
| Cause of ESRD | FSGS | CGN | CGN |
| Pretransplant serum creatinine (mg/dL) | 11.53 | 14.58 | 10.18 |
| Hemodialysis time (months) | 120 | 5 | 6 |
| Immunological evaluation | |||
| HLA mismatch | 3 | 2 | 2 |
| Crossmatch test | Negative | Negative | Negative |
| Anti‐HLA DSAs | None | None | None |
Figure 1.
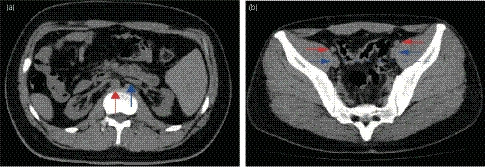
(a) CT image shows mirror‐image location of the abdominal organs and bilateral atrophic kidneys. Ventral aorta (red arrow) and inferior vena cava (blue arrow) are reversed left and right. (b) The external iliac arteries (red arrows) and veins (blue arrows) are shown. Right side vessels are deeper than left side ones (blue dashed line) (Case 3).
Table 2.
Surgical and postoperative outcomes
| Variables | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Procurement side of donor kidney | Left | Left | Left |
| Weight of donor kidney (g) | 222 | 140 | 182 |
| Transplant side of iliac fossa | Right | Right | Right |
| Warm ischemic time (min) | 5 | 4 | 5 |
| Total ischemic time (min) | 86 | 108 | 90 |
| Total operation time (min) | 241 | 310 | 274 |
| Estimated blood loss (mL) | 80 | 290 | 220 |
| Blood transfusion (yes/no) | Yes | Yes | Yes |
| First urine time (min) | 16 | N/A | 5 |
| Perioperative complication | None | None | None |
| Postoperative complication | Congestive kidney | None | None |
| Follow‐up period (months) | 17 | 14 | 5 |
| Posttransplant serum creatinine (mg/dL) | 1.12 | 1.19 | 1.32 |
N/A, not available.
Figure 2.
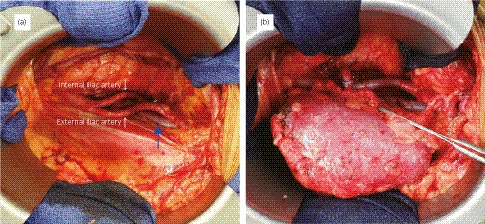
(a) Intraoperative view of the right iliac fossa before blood vessel anastomosis. The right external iliac vein (blue arrow) was deeper than the normal anatomy. (b) Intraoperative view after KT. The course of the renal vein was slightly in the vertical direction (indicated with forceps) (Case 1).
In case 1, the patient developed oliguria with hematuria and proteinuria on day 2 after transplantation. Ultrasonography showed swelling of the transplanted kidney. Doppler examination revealed absent diastolic flow. MRI demonstrated no obvious renal vein thrombosis. At first, acute rejection was suspected, the patient received plasma exchange, intravenous immunoglobulin, and steroid pulse therapy. He required two sessions of hemodialysis, and recovery was achieved on day 10. Then, renal biopsy was performed, but it was not diagnosed as rejection. We speculated that the compression of postoperative edema of connective tissue changed the position of the transplanted kidney, and the renal vein which was anastomosed at a deeper position than the normal anatomical case was kinked. Nevertheless, the renal function of all patients improved, and they could be weaned off the dialysis. The follow‐up period was 5–17 months, renal function was stable, and protocol biopsy at 3 and 12 months posttransplant showed no rejection in all cases.
Discussion
Although some cases of nephrotic syndrome in SI totalis patients have been reported,4, 5, 6 the mechanisms of renal dysfunction in adults with SI totalis are still unknown. Domański et al. hypothesized that the underlying mechanism for ESRD was the disturbance of the transforming growth factor‐β and Smad protein signaling because of both nephritic syndrome and SI totalis.6 Some cases of renal amyloidosis in Kartagener's syndrome combined with SI, chronic sinusitis, and bronchiectasis have been reported;7, 8 however, the cases presented only had SI. In our report, cases 1 and 2 were siblings, which suggests that some genetic abnormalities may have influenced renal dysfunction, leading to ESRD.
Because of the rarity of SI totalis, cases of SI totalis in ESRD patients who underwent KT are not well reported. Katsika et al. reported renal transplantation in SI totalis patients, but the reports focused on anesthetic management,9 and no studies about KT surgery for ESRD patients with SI totalis and its postoperative course have been reported. To our knowledge, this is the first case series of living‐donor KT recipients with SI totalis.
During KT, the donor kidney is preferably placed in the opposite iliac fossa so that the collecting system will be most anterior; this displaces the ureter away from the iliac vessels, providing more accessibility for venous anastomosis.10 Commonly, the left renal vein of the donor kidney is longer than the right one, right iliac vessels are at a more superficial position, and the right external iliac vein has a more horizontal course than the left. Therefore, the left donor kidney is commonly transplanted into the right iliac fossa. In our cases, the donor kidneys were left kidneys and were transplanted in the right iliac fossa, but the recipients were SI totalis patients, so the external iliac veins were deeper than in the normal anatomy. This was probably associated with the congestive kidney in case 1, which was caused by tension or kinking of the renal vein, and thus the need to cut off the internal iliac vein for venous anastomosis in case 3. Posttransplant kidney functions of all cases were stable with no recurrence during follow‐up.
In conclusion, KT for ESRD patients with SI totalis is the same as normal KT except that attention is paid to venous anastomosis. However, given the rarity of SI totalis, further studies are required to examine higher number of patients with a longer follow‐up period.
Disclosure
The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of Kameda medical center, Approval No. 18‐141. All informed consent was obtained from the subjects.
Conflict of interest
The authors declare no conflict of interest.
References
- 1. Levin M. The embryonic origins of left‐right asymmetry. Crit. Rev. Oral Biol. Med. 2004; 15: 197–206. [DOI] [PubMed] [Google Scholar]
- 2. Huang SC, Chen WJ. Renal dysplasia and situs inversus totalis: an autopsy case report and literature review. Chang Gung Med. J. 2000; 23: 43–7. [PubMed] [Google Scholar]
- 3. Kaynar K, Ulusoy S, Gul S, Ozkan G, Caylan R, Kosucu P. Renal hypoplasia and situs inversus totalis. Nephrology 2005; 10: 189–91. [DOI] [PubMed] [Google Scholar]
- 4. Vikrant S, Kumar S, Raina R, Sharma A. Nephrotic syndrome in a patient with situs inversus totalis. Clin. Exp. Nephrol. 2008; 12: 215–8. [DOI] [PubMed] [Google Scholar]
- 5. More VB, Saxena A, Sharma R. Nephrotic syndrome and situs inversus – a chance association? Saudi J. Kidney Dis. Transpl. 2016; 27: 177–8. [DOI] [PubMed] [Google Scholar]
- 6. Domanski M, Domanski L, Ciechanowski K. Nephrotic syndrome and situs inversus viscerum: correlation or coincidence. J. Nephrol. 2005; 18: 623–5. [PubMed] [Google Scholar]
- 7. Osman EM, Abboud OI, Sulaiman SM, Musa AR, Beleil OM, Sharfi AA. End‐stage renal failure in Kartagener's syndrome. Nephrol. Dial. Transplant. 1991; 6: 747. [DOI] [PubMed] [Google Scholar]
- 8. El Houssni S, Laine M, Bziz A, Alhamany Z, Eddine Bourkadi J, Bayahia R. Renal amyloidosis revealing a Kartagener's syndrome. Nephrol. Ther. 2015; 11: 50–2. [DOI] [PubMed] [Google Scholar]
- 9. Katsika E, Aslanidis T, Charitidou S. Renal transplantation in a patient with Bardet‐Biedl syndrome, situs inversus totalis and bifid epiglottis: anesthetic management. Hippokratia 2011; 15: 376. [PMC free article] [PubMed] [Google Scholar]
- 10. Goldfarb DA, Flechner SM, Modlin CS. Renal transplantation In: Novick AC, Jones JS (eds). Operative Urology at the Cleveland Clinic. Humana Press, Totowa, 2006; 121–32. [Google Scholar]


