Abstract
Aim
Clinical trials and meta‐analyses have demonstrated the efficacy of high‐frequency repetitive transcranial magnetic stimulation (rTMS) over the left prefrontal cortex in treatment‐resistant depression. The aim of this study was to prospectively evaluate the effectiveness of the conventional 37.5‐minute vs 18.75‐minute rTMS protocol over the left prefrontal cortex in patients with treatment‐resistant depressive episode.
Methods
Thirty patients with treatment‐resistant depression or bipolar disorder depressive episode were randomized 1:1 to either 37.5‐minute or 18.75‐minute rTMS protocol groups. rTMS treatment was applied at 120% resting motor threshold with 10 Hz over the left prefrontal cortex. Treatment sessions were delivered for a total of 3000 pulses/d, 5 days a week, for 4‐6 weeks. Patients received a 75 trains with “4 sec on and 26 sec off” for 37.5 minutes or a 75 trains with “4 sec on and 11 sec off” for 18.75 minutes. Severity of depression was rated with the Quick Inventory of Depressive Symptomatology (QIDS) and Patient Health Questionnaire (PHQ‐9). Remission was defined as a total score of 5 or less on the QIDS. The primary outcome measure was to compare the remission rate between the both groups.
Results
Thirteen of 30 patients (43.3%) showed remission at week 6. There were no significant differences in the remission rate between the conventional 37.5‐ and 18.75‐minute protocol groups (46.7% and 40.0%, respectively). No seizures or treatment‐emergent mania/hypomania were occurred.
Conclusion
These findings suggest that, compared with the conventional one, rTMS with 18.75‐minute protocol might be equally effective and clinically beneficial in saving the treatment session length. Further well‐designed studies are needed.
Keywords: depression, PHQ‐9, Quick Inventory of Depressive Symptomatology, repetitive transcranial magnetic stimulation, stimulation protocol
Effectiveness of rTMS in treatment‐resistant depression: a randomized clinical trial of 37.5‐minute vs 18.75‐minute protocol
![]()
1. INTRODUCTION
Among psychiatric disorders, depression is common and costly, disabling various social functions.1, 2, 3, 4 There are a number of antidepressant medications available, but some depressed patients do not respond to them.5 Therefore, more powerful therapeutic options are needed. For patients with treatment‐resistant depression, repetitive transcranial magnetic stimulation (rTMS) is an emerging option.
Repetitive transcranial magnetic stimulation is a noninvasive technique that stimulates directly cortex and modulates cortical and subcortical excitability.6, 7, 8, 9 In Japan, rTMS was approved as a novel therapy for treatment‐resistant depression in September 2017. Clinical trials and meta‐analyses have demonstrated the efficacy of facilitatory stimulation such as high‐frequency rTMS and intermittent theta burst stimulation (iTBS) over the left prefrontal cortex.10, 11, 12, 13, 14, 15
The conventional rTMS protocol with 10 Hz approved in Japan and other countries requires 37.5 minutes per treatment session,11, 12, 16 because one train including “4 sec on and 26 sec off” is repeated 75 times per treatment session with 3000 pulses. In terms of the cost‐effectiveness, to reduce a treatment session length is needed in clinical practice.
In this preliminary study, we sought to compare directly the conventional 37.5‐minute protocol with “4 sec on and 26 sec off” and 18.75‐minute protocol with “4 sec on and 11 sec off.” In the latter protocol, one train including “4 sec on and 11 sec off” is repeated 75 times per treatment session with 3000 pulses. Therefore, this rTMS protocol can shorten the treatment session length by half. This is the first study to prospectively evaluate the effectiveness of the conventional 37.5‐minute vs 18.75‐minute rTMS protocol over the left prefrontal cortex in patients with treatment‐resistant depressive episode.
2. METHODS
2.1. Subjects
Eligible subjects were patients aged 25‐75 with a DSM‐5 diagnosis of major depressive disorder or bipolar disorder depressive episode. Patients who had a total score of >11 on the Quick Inventory of Depressive Symptomatology (QIDS)17 and did not respond to adequate medications in the current episode were enrolled in this study.
Exclusion criteria for this study included psychotic features in the current episode; schizophrenia or schizoaffective disorder; a substance abuse or dependence; depression due to a medicine or substance; severe neurological disorders; any increased risk of seizure including a personal or close family history of convulsion; active suicidal ideation; a presence of ferromagnetic material in the upper body; and pregnancy. Medications given to the patients were not allowed to have changed in the 4 weeks before the start of the first rTMS treatment or during the trial.
2.2. Overview
This study with a randomized open‐label trial was conducted at Medicalcare Toranomon Clinic in Tokyo. The study protocol was approved by the Ethics Committee of the Japanese Association of Rework for Depression. All patients provided written informed consent before undergoing any study procedures. The enrolled patients were randomized 1:1 to either the conventional 37.5‐minute or 18.75‐minute rTMS protocol groups.
2.3. Intervention
Repetitive transcranial magnetic stimulation was administered using a MagPro R30 magnetic stimulator and a Cool‐B65 coil (MagVenture A/S). rTMS treatment was applied at 120% resting motor threshold (MT) with 10 Hz over the left prefrontal cortex. The standardized stimulation site was over the left prefrontal cortex and was determined by movement of the rTMS coil 5 cm anterior to the MT location. Treatment sessions were delivered for a total of 3000 pulses/d, 5 days a week, for 4‐6 weeks. In the conventional rTMS protocol, a 75 trains with “4 sec on and 26 sec off” lasted for 37.5 minutes with 3000 pulses (Figure 1). In the other protocol, a 75 trains with “4 sec on and 11 sec off” lasted for 18.75 minutes with 3000 pulses (Figure 1).
Figure 1.
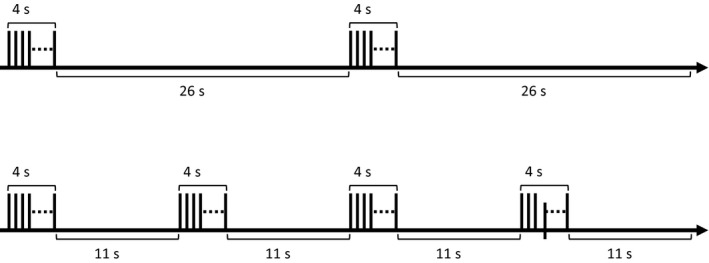
rTMS protocol delivered to patients in this trial. In the upper 37.5‐min protocol, a train including “4 sec on and 26 sec off” is repeated 75 times per treatment session. In the lower protocol, a 75 trains with “4 sec on and 11 sec off” lasts for 18.75 min. The number of stimulation pulse is the same in the both protocols
2.4. Clinical assessments
The severity of depression was rated with the QIDS and Patient Health Questionnaire (PHQ‐9).18, 19 The Young Mania Rating Scale (YMRS)20 was used to evaluate a manic symptom. Remission was defined as a total score of 5 or less on the QIDS. Response was defined as a 50% or greater reduction in the QIDS score. The QIDS, PHQ‐9, and YMRS were rated every 2 weeks at baseline, week 2, week 4, and week 6. The primary outcome measure was to compare the remission rate between the both groups.
2.5. Statistical analysis
In demographic and clinical characteristics of patients, differences between the 37.5‐ and 18.75‐minute protocol groups were analyzed, respectively, using independent t tests. Total scores of QIDS and PHQ‐9 at baseline, week 2, week 4, and week 6 were analyzed individually using independent t tests for the differences between the 37.5‐ and 18.75‐minute protocol groups. Two‐way repeated‐measures analysis of variance (ANOVA) was used to compare changes in the QIDS and PHQ‐9 scores and to estimate the main effect of group as the between‐subjects factor (37.5‐ and 18.75‐minute protocol groups) and the main effect of time as the within‐subjects (baseline, week 2, week 4, and week 6). Chi‐square test was used to compare the remission and response rates between the 37.5‐ and 18.75‐minute protocol groups. Statistical analysis was conducted using the IBM SPSS Statistics 20.0 (IBM Corporation, Armonk, New York), with the level of statistical significance set at P < 0.05.
3. RESULTS
Thirty patients entered the study, and 28 patients completed the entire study. Two patients did not complete the study, because one patient allocated into the 37.5‐minute protocol group did not visit an outpatient clinic after the sixth treatment session, and another allocated into the 18.75‐minute group did not visit after the ninth treatment session.
Demographic and clinical characteristics of patients were shown in Table 1. There were no significant differences between the 37.5‐ and 18.75‐minute protocol groups in age at onset, number of episodes, duration of current episode, QIDS score, and PHQ‐9 score at baseline except the age of patients (Table 1). There were also no significant differences between the both groups in the QIDS and PHQ‐9 scores at baseline, week 2, week 4, and week 6, respectively (Table 2). For changes in the QIDS and PHQ‐9 scores in the both groups, two‐way repeated‐measures ANOVA showed a significant main effect of time (QIDS: F (3, 78) = 44.75, P < 0.001; PHQ‐9: F (3, 78) = 36.40, P < 0.001). Multiple comparisons using the Bonferroni correction showed that the total scores on the QIDS and PHQ‐9 in the both groups decreased significantly from the baseline score to the week 6 score, respectively (P < 0.001). There was no significant main effect of group (QIDS: F (1, 26) = 0.28, P = 0.60; PHQ‐9: F (1, 26) = 0.09, P < 0.77).
Table 1.
Demographic and clinical characteristics of patients receiving 37.5‐min or 18.75‐min rTMS protocol
|
37.5‐min protocol |
18.75‐min protocol |
Statistical analysis | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | P | |
| Age (y) | 49.6 | 7.5 | 42.9 | 9.1 | 2.19 | 28 | 0.04 |
| Age at onset (y) | 37.5 | 8.9 | 34.1 | 7.4 | 1.12 | 28 | 0.27 |
| Depression type (MDD, BD) | 10, 5 | 9, 6 | |||||
| Number of depressive episodes | 3.1 | 1.0 | 3.2 | 1.7 | −0.26 | 28 | 0.80 |
| Number of manic/hypomanic episodes | 0.6 | 1.1 | 0.7 | 1.2 | −0.32 | 28 | 0.75 |
| Duration of current depressive episode (mo) | 9.6 | 6.1 | 10.3 | 8.4 | −0.25 | 28 | 0.81 |
| QIDS score at baseline | 13.5 | 2.6 | 14.2 | 2.9 | −0.72 | 28 | 0.48 |
| PHQ‐9 score at baseline | 14.9 | 3.8 | 14.5 | 5.1 | 0.29 | 28 | 0.78 |
| Treatment sessions | 27.3 | 6.1 | 27.1 | 6.8 | 0.09 | 28 | 0.93 |
Depression type was analyzed using chi‐square test (χ 2 = 0.14, df = 1, P = 0.71).
Abbreviations: BD, bipolar disorder; MDD, major depressive disorder; PHQ‐9, patient health questionnaire; QIDS, quick inventory of depressive symptomatology.
Table 2.
Changes in QIDS and PHQ‐9 scores in patients: 37.5‐min vs 18.75‐min rTMS protocol
|
37.5‐min protocol |
18.75‐min protocol |
Statistical analysis | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | P | |
| QIDS | |||||||
| Baseline | 13.5 | 2.6 | 14.2 | 2.9 | −7.23 | 28 | 0.48 |
| Week 2 | 9.3 | 3.8 | 9.9 | 4.7 | −4.00 | 26 | 0.69 |
| Week 4 | 7.7 | 4.1 | 8.3 | 5.6 | −0.31 | 26 | 0.76 |
| Week 6 | 6.1 | 4.0 | 7.3 | 5.7 | −0.61 | 26 | 0.55 |
| PHQ‐9 | |||||||
| Baseline | 14.9 | 3.8 | 14.5 | 5.1 | 0.29 | 28 | 0.78 |
| Week 2 | 9.7 | 4.3 | 10.6 | 5.8 | −0.48 | 26 | 0.64 |
| Week 4 | 8.5 | 4.3 | 9.7 | 6.6 | −0.58 | 26 | 0.57 |
| Week 6 | 7.2 | 4.5 | 7.9 | 7.3 | −0.31 | 26 | 0.76 |
Abbreviations: PHQ‐9, patient health questionnaire; QIDS, quick inventory of depressive symptomatology.
The number of patients showing remission in the 37.5‐ and 18.75‐minute protocol groups was 7 (46.7%) and 6 (40.0%) at week 6, respectively (Table 3). Of seven patients with remission, 3 were major repressive disorder and 4 were bipolar depression. Of six patients, 3 were major depressive disorder and 3 were bipolar depression. The number of responders in the 37.5‐ and 18.75‐minute protocol groups was 9 (60.0%) and 7 (46.7%) at week 6, respectively (Table 3). Of nine patients with response, 5 were major depressive disorder and 4 were bipolar depression. Of 7 patients, 4 were major depressive disorder and 3 were bipolar depression. There were no significant differences in the remission and response rates at week 6 between the both groups (Table 3). There were also no significant differences in the remission and response rates between major depressive disorder and bipolar depression (remission: χ 2 = 2.92, df = 1, P = 0.09; response: χ 2 = 0.74, df = 1, P = 0.39).
Table 3.
Patients with remission and response at week 6 following 37.5‐min or 18.75‐min rTMS protocol
|
37.5‐min protocol |
18.75‐min protocol |
Statistical analysis | |||
|---|---|---|---|---|---|
| N = 15 | N = 15 | χ 2 | df | P | |
| Number of patients with remission | 7 | 6 | 0.14 | 1 | 0.71 |
| Number of patients with response | 9 | 7 | 0.54 | 1 | 0.46 |
Two patients who did not complete the entire study were nonremitters and nonresponders.
As to side effects, there were no severe adverse events like a seizure or treatment‐emergent mania/hypomania during the trial. Ten patients (10/30, 33.3%) had stimulation pain or discomfort. Of 10 patients, 7 were included in the 37.5‐minute protocol group. There were no significant differences between the both groups (χ 2 = 2.40, df = 1, P = 0.12).
4. DISCUSSION
This is the first study to directly compare as well as to prospectively evaluate the effectiveness of the conventional 37.5‐minute vs 18.75‐minute rTMS protocol in treatment‐resistant depressive episode. The main outcome of this study was that there were no significant differences in the remission and response rates between the both groups. The scores on the QIDS and PHQ‐9 in the both groups were significantly decreased after the rTMS treatment sessions. Additionally, there were no severe adverse events during the trial.
Two large randomized clinical trials (RCTs) showed that remission rate ranged from 14.1% with active and 5.1% with sham rTMS (HAMD24),12 to 14.2% with active and 5.5% with sham rTMS (MADRS),11 15.5% with active and 8.9% with sham rTMS (HAMD17),11 and 17.4% with active and 8.2% with sham rTMS (HAMD24).11 HAMD and MADRS indicate Hamilton Depression Rating Scale and Montgomery‐Åsberg Depression Rating Scale. According to a meta‐analysis of RCTs, the remission rates in the active and sham stimulation were 18.6% and 5.0%.21 In the current study with an open‐label trial, the remission rates were 46.7% with 37.5‐minute and 40.0% with 18.75‐minute protocol. Those remission rates are superior to ones from the results of previous two RCTs11, 12 and meta‐analysis of RCTs.21 In clinical practice without a sham control, clinician‐assessed remission rate was 37.1%,22 and patient‐reported remission rate ranged from 28.7 (PHQ‐9) to 26.5% (Inventory of Depressive Symptoms—Self Report, IDS‐SR).22 We used the QIDS and PHQ‐9 to rate the severity of depression in this study. About 43% of the patients in the 37.5‐ and 18.75‐minute protocol groups showed remission after the 6‐week rTMS treatment. These remission rates are slightly superior to those of the previous report.22 The differences in the remission rate can be explained by a study design, such as double‐blind trial or an open‐label trial.
The current study has some limitations to be considered. One limitation is the study design without a sham control in an open‐label trial. Secondly, patients included not only major depression but also bipolar disorder depressive episode. We sought to directly compare the conventional 37.5‐ and 18.75‐minute rTMS protocol in this study. Technically, to test the efficacy or to evaluate the effectiveness of the 18.75‐minute protocol, further confirmatory studies are needed with a single population not heterogenous depression, such as a randomized sham‐controlled trial or a randomized noninferiority trial compared with the 37.5‐minute protocol. Additionally, we delivered rTMS treatments in the both groups in combination with medical treatments. However, to lessen the effects of medications on the outcomes, the medications given to the patients were not allowed to have changed during the trial, and rTMS treatments in the both groups were administered under the same condition.
The findings of this preliminary study revealed that rTMS with the both protocols relieved safely and tolerably depressive symptoms, suggesting that, compared with the conventional one, rTMS with 18.75‐minute protocol might be equally effective and clinically beneficial in saving the treatment session length. Further well‐designed studies are needed.
CONFLICT OF INTEREST
SK reports research grants, speaking fees, or advisory board work with Century Medical, Inter‐Riha, Teijin Pharma, and Vorpal Technologies. The other authors have no financial disclosures or conflicts of interest to report.
AUTHOR CONTRIBUTION
SK contributed to conception and design of the study, acquisition of data, and drafting the manuscript, tables, and figures. MM and HN contributed to acquisition and monitoring of data. YM and RY contributed to design of the study and reviewing the manuscript. TO and YI contributed acquisition of data and reviewing the manuscript. SK and YI had full access to all the data in the study and take responsibility for integrity of data and accuracy of analysis. All the authors approved the final manuscript.
DATA REPOSITORY
The authors provided the raw data of all the patients in this study as Supporting information.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
This study protocol was approved by the Ethics Committee of the Japanese Association of Rework for Depression.
INFORMED CONSENT
All the patients of this study provided written informed consent before undergoing any study procedures.
Supporting information
Kito S, Miyazi M, Nakatani H, et al. Effectiveness of high‐frequency left prefrontal repetitive transcranial magnetic stimulation in patients with treatment‐resistant depression: A randomized clinical trial of 37.5‐minute vs 18.75‐minute protocol. Neuropsychopharmacol Rep. 2019;39:203–208. 10.1002/npr2.12066
Clinical Trial Registration: UMIN‐CTR, UMIN000032312.
REFERENCES
- 1. Ebmeier KP, Donaghey C, Steele JD. Recent developments and current controversies in depression. Lancet. 2006;367:153–67. [DOI] [PubMed] [Google Scholar]
- 2. Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–53. [DOI] [PubMed] [Google Scholar]
- 3. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370:851–8. [DOI] [PubMed] [Google Scholar]
- 4. Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray C, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. [DOI] [PubMed] [Google Scholar]
- 6. Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. 2007;8:559–67. [DOI] [PubMed] [Google Scholar]
- 8. Kito S, Fujita K, Koga Y. Changes in regional cerebral blood flow after repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex in treatment‐resistant depression. J Neuropsychiatry Clin Neurosci. 2008;20:74–80. [DOI] [PubMed] [Google Scholar]
- 9. Kito S, Hasegawa T, Koga Y. Neuroanatomical correlates of therapeutic efficacy of low‐frequency right prefrontal transcranial magnetic stimulation in treatment‐resistant depression. Psychiatry Clin Neurosci. 2011;65:175–82. [DOI] [PubMed] [Google Scholar]
- 10. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. 2013;26:13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–16. [DOI] [PubMed] [Google Scholar]
- 12. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham‐controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–16. [DOI] [PubMed] [Google Scholar]
- 13. Lefaucheur J‐P, André‐Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence‐based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2014;125:2150–206. [DOI] [PubMed] [Google Scholar]
- 14. Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta‐analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–84. [DOI] [PubMed] [Google Scholar]
- 15. Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta‐analysis. JAMA Psychiatry. 2017;74:143–52. [DOI] [PubMed] [Google Scholar]
- 16. Perera T, George MS, Grammer G, Janicak PG, Pascual‐Leone A, Wirecki TS. The clinical tms society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16‐Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS‐C), and self‐report (QIDS‐SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. [DOI] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muramatsu K, Miyaoka H, Kamijima K, et al. The Patient Health Questionnaire, Japanese version: validity according to the Mini‐International Neuropsychiatric Interview‐Plus. Psychol Rep. 2007;101:952–60. [DOI] [PubMed] [Google Scholar]
- 20. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. [DOI] [PubMed] [Google Scholar]
- 21. Berlim MT, van den Eynde F, Tovar‐Perdomo S, Daskalakis ZJ. Response, remission and drop‐out rates following high‐frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta‐analysis of randomized, double‐blind and sham‐controlled trials. Psychol Med. 2014;44:225–39. [DOI] [PubMed] [Google Scholar]
- 22. Carpenter LL, Janicak PG, Aaronson ST, Boyadjis T, Brock DG, Cook IA, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


