Abstract
Background
Aluminum (Al) is considered to be a neurotoxic metal, and excessive exposure to Al has been reported to be a potential risk factor for neurodegenerative diseases. Al ammonium sulfate is one of the Al compounds that is widely used as a food additive. However, the effects of the oral administration of Al ammonium sulfate on physical development and behavior remain to be examined.
Methods
In this study, we investigated the effects of the administration of Al ammonium sulfate 12‐water dissolved in drinking water (0.075 mg/mL) beginning in adolescence on various types of behavior in adult female C57BL/6J mice through a battery of behavioral tests (low‐dose experiment; Experiment 1). We further examined the behavioral effects of the oral administration of a higher dose of the Al compound in drinking water (1 mg/mL) beginning in the prenatal period on behavior in adult male and female mice (high‐dose experiment; Experiment 2).
Results
In the low‐dose experiment, in which females’ oral intake of Al was estimated to be 0.97 mg Al/kg/d as adults, Al‐treated females exhibited an increase in total arm entries in the elevated plus maze test, an initial decrease and subsequent increase in immobility in the forced swim test, and reduced freezing in the fear conditioning test approximately 1 month after the conditioning session compared with vehicle‐treated females (uncorrected P < .05). However, the behavioral differences did not reach a statistically significant level after correction for multiple testing. In the high‐dose experiment, in which animals’ oral intakes were estimated to be about ten times higher than those in the low‐dose experiment, behavioral differences found in the low‐dose experiment were not observed in high‐dose Al‐treated mice, suggesting that the results of the low‐dose experiment might be false positives. Additionally, although high‐dose Al‐treated females exhibited increased social contacts with unfamiliar conspecifics and impaired reference memory performance, and high‐dose Al‐treated mice exhibited decreases in prepulse inhibition and in correct responses in the working memory task (uncorrected P < .05), the differences in any of the behavioral measures did not reach the significance level after correction for multiple testing.
Conclusion
Our results show that long‐term oral exposure to Al ammonium sulfate at the doses used in this study may have the potential to induce some behavioral changes in C57BL/6J mice. However, the behavioral effects of Al were small and statistically weak, as indicated by the fact that the results failed to reach the study‐wide significance level. Thus, further study will be needed to replicate the results and reevaluate the behavioral outcomes of oral intake of Al ammonium sulfate.
Keywords: aluminum ammonium sulfate, behavioral test battery, C57BL/6J, mice, oral intake
Aluminum (Al) ammonium sulfate was orally administered to C57BL/6J mice (estimated dose of 0.97‐9.78 Al mg/kg/d). Behavioral effects of Al were assessed in a battery of behavioral tests in mice in adulthood. Statistically significant behavioral differences were not found between Al‐ and vehicle‐treated mice.

1. INTRODUCTION
Aluminum (Al) is one of the most abundant metallic elements in the earth's crust and is widely present in soil, air, water, and plants. Al compounds are extensively used for various purposes in food, beverages, cosmetics, and medicines. Exposure to Al from all sources, including natural sources, water consumption, and food materials, is estimated to be in the range of 10‐140 mg/wk (0.2‐2.3 mg/kg/wk, assuming a body weight of 60 kg) in adult populations.1 Currently, the provisional tolerable weekly intake (PTWI) is set at 2 mg/kg/wk based on a no‐observed‐adverse‐effect level of 30 mg/kg/d from findings in animal studies.1 Numerous epidemiologic studies have reported that excessive exposure to Al is both neurotoxic and a potential risk factor for neurodegenerative conditions such as Alzheimer's disease,2, 3, 4 amyotrophic lateral sclerosis,5, 6 and Parkinson's disease,7, 8 although the causal relationships between Al exposure and these conditions, especially Alzheimer's disease, remain controversial.9, 10, 11
Animal studies have been performed to determine whether exposure to Al compounds such as aluminum chloride, aluminum nitrate, and aluminum lactate at different developmental stages affects the brain and behavior. Perinatal and/or adult exposure to Al compounds has been reported to induce behavioral changes such as reduced locomotor activity, impaired active and passive avoidance behaviors, and spatial memory deficits in rodents.12, 13, 14, 15, 16, 17, 18, 19 In contrast, multiple studies report that Al exposure has no such behavioral effects.20, 21, 22, 23, 24, 25 The differences in the severity of the effects shown in these studies might be attributable to methodological differences, for example, differences in the form of the Al, the dose, the administration route, the exposure duration, and the behavioral testing procedure.
Aluminum ammonium sulfate is one of the Al compounds that is widely used in food additives and beverages as a buffering and neutralizing agent to maintain acidity during food processing, water purification, and color fixation.26, 27, 28 However, the effects of oral exposure to Al ammonium sulfate on physical development and behavior have not been examined. In this study, to address the lack of information regarding the behavioral effects of Al ammonium sulfate, we investigated the effects of the oral administration of Al ammonium sulfate dissolved in drinking water at a relatively low dose (0.075 mg/mL) beginning in adolescence on the behavior of adult female C57BL/6J mice using a battery of behavioral tests, including a general health and neurological screen, light/dark transition, open field, elevated plus maze, hot plate, social interaction, rotarod, three‐chamber social approach, startle response/prepulse inhibition, Porsolt forced swim, T‐maze spontaneous alternation, Y‐maze, Barnes maze, tail suspension, fear conditioning, and home‐cage social interaction tests (Experiment 1). Next, we further explored the behavioral effects of the oral administration of Al ammonium sulfate in drinking water at a relatively high dose (1 mg/mL) beginning in the prenatal period in adult male and female C57BL/6J mice (Experiment 2). This study provides novel information about effects of oral exposure to aluminum ammonium sulfate at the doses used on a variety of behaviors in the behavioral tests that had not been previously studied in mice.
2. MATERIALS AND METHODS
2.1. Animals
Male and female C57BL/6J mice were used. The general procedure of the experiments is illustrated in Figure 1. In Experiment 1, 40 naïve females were transported from the Charles River Laboratories Japan to our animal facility at the age of 25 days. After their arrival, females were group‐housed (4 per cage) in a plastic cage (22.7 × 32.3 × 12.7 cm) in a room with a 12‐hour light/dark cycle (lights on at 7:00 am). The room temperature was maintained at 23 ± 2°C. The females in each cage were randomly assigned to either the vehicle‐treated group (n = 20) or the low‐dose Al‐treated group (dose of 0.075 mg/mL, n = 20). The mice in the Al‐treated group were allowed to freely drink a solution containing Al ammonium sulfate from 4 weeks of age 3 days after arrival, as described below. Their body weights were measured every 2 weeks, and their behaviors were assessed with a battery of behavioral tests after 9 weeks of age (Table 1). In Experiment 2, twenty pregnant females, which were transported from the Charles River Laboratories Japan 6 days after finding a vaginal plug (Day 0 = day of pregnancy), were singly housed in a plastic cage after arrival in our animal facility. The pregnant females were randomly assigned to either the vehicle‐treated group (n = 10) or the high‐dose Al‐treated group (dose of 1 mg/mL, n = 10). From Day 11 of pregnancy, females in the high‐dose Al‐treated group were given Al ammonium sulfate in drinking water at the dose of 1 mg/mL. The cages were checked every morning for the presence of pups. On the evening of the day of parturition (postpartum Day 0) and postpartum days 7, 14, and 21, both litter size and pup body weights were recorded. On postpartum Day 21, the offspring were weaned and housed in same‐sex groups of 4 animals per cage. In each treatment group, 20 male and 20 female offspring were used in the behavioral experiments. After weaning, the offspring from high‐dose Al‐treated dams were given Al in their drinking water at the dose of 1 mg/mL in the same manner throughout the experiments. From the age of 9 weeks, the male and female offspring were subjected to a battery of behavioral tests (Table 1). All of the animals were provided with food (CRF‐1, Oriental Yeast Co., Ltd., which would contain 0.21 mg Al/g) ad libitum throughout the experiments. All of the experimental procedures were approved by the Institutional Animal Care and Use Committee of Kobe University (# P110604‐R1) and of Fujita Health University (#I0741).
Figure 1.
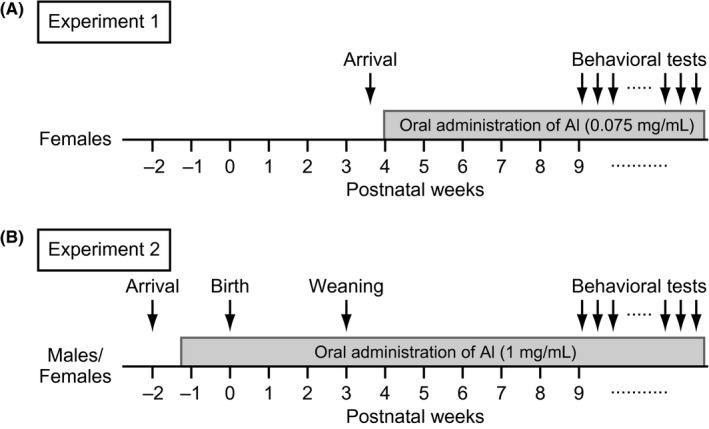
Schematic diagram of experimental procedures. Flow diagram of the experimental design for (A) Experiment 1 and (B) Experiment 2
Table 1.
A behavioral test battery of aluminum‐treated mice
| Order | Test | Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|---|---|
| Age | Period | Table/figure | Age | Period | Table/figure | ||
| 1 | General health and neurological screen | 10 wk (2 mo) | 6 wk | Table S2 | 9‐10 wk (2 mo) | 11 wk | Table S2 |
| 2 | Light/dark transition test | 10 wk (2 mo) | 6 wk | Figure 2A‐D | 10‐11 wk (2 mo) | 11‐12 wk | Figure 5A‐D |
| 3 | Open field test | 10 wk (2 mo) | 6 wk | Figure S2A‐D | 13‐14 wk (3 mo) | 14‐15 wk | Figure S2E‐H |
| 4 | Elevated plus maze test | 11 wk (2 mo) | 7 wk | Figure 2E‐H | 15‐16 wk (3 mo) | 17 wk | Figure 5E‐H |
| 5 | Hot plate test | 11 wk (2 mo) | 7 wk | Table S2 | 16‐17 wk (3 mo) | 18 wk | Table S2 |
| 6 | Social interaction test | 11 wk (2 mo) | 7 wk | Figure 3A‐E | 17 wk (3‐4 mo) | 18 wk | Figure 6A‐E |
| 7 | Rotarod test | 12 wk (2 mo) | 8 wk | Table S2 | 18‐19 wk (4 mo) | 20 wk | Table S2 |
| 8 | Three‐chamber social approach test | 14 wk (3 mo) | 10 wk | Figure 3F‐I | 22‐23 wk (5 mo) | 24‐25 wk | Figure 6F‐I |
| 9 | Startle response/prepulse inhibition tests | 15 wk (3 mo) | 11 wk | Table S2 | 26 wk (6 mo) | 27 wk | Table S2 |
| 10 | Porsolt forced swim test | 15 wk (3 mo) | 11 wk | Figure 2I | 32‐33 wk (7 mo) | 33‐34 wk | Figure 5I |
| 11 | T‐maze spontaneous alternation test | 16 wk (3 mo) | 12 wk | Figure 4A | 63‐65 wk (14 mo) | 65‐66 wk | Figure 7A |
| 12 | Y‐maze test | 19 wk (4 mo) | 15 wk | Figure 4B | NT | ||
| 13 | Barnes maze test | 26‐32 wk (6‐7 mo) | 22‐28 wk | Figures 4C, S3A‐E | 87‐96 wk (20‐22 mo) | 88‐97 wk | Figures 7B, S3F‐J |
| 14 | Tail suspension test | 33 wk (7 mo) | 29 wk | Figure 2J | 93‐97 wk (21‐22 mo) | 94‐98 wk | Figure 5J |
| 15 | Fear conditioning test | 33‐37 wk (7‐8 mo) | 29‐33 wk | Figures 4D‐H, S4A | 95‐102 wk (21‐23 mo) | 96‐103 wk | Figures 7C‐G, S4B |
| 16 | Home‐cage social interaction test | 37‐38 wk (8 mo) | 33‐34 wk | Figure 3J, K | 106‐108 wk (24 mo) | 107‐109 wk | Figure 6J, K |
wk, week; mo, month; NT, not tested; Period, administration period.
The administration period in Experiment 2 includes a period exposed to aluminum via pregnant females during the prenatal period.
2.2. Treatment with aluminum in drinking water
Animals in the Al‐treated group were given Al ammonium sulfate 12‐water, 99.9% (AlNH4(SO4)2·12H2O, FW: 453.33, Wako Pure Chemical Industries, Ltd.) dissolved in drinking water from a bottle. The vehicle‐treated group was provided with ammonium sulfate ((NH4)2SO4, FW: 132.14, Wako Pure Chemical Industries, Ltd.) in their drinking water. In our pilot experiments, a few pregnant C57BL/6J mice died after daily oral administration of the Al compound dissolved in drinking water at a dose of 0.1 or 1 mg/mL. In addition, female pups exposed to the Al compound at the lower dose of 0.075 mg/mL during the prenatal and postnatal period through dams had a lower body weight than vehicle‐treated female pups (see Data S1). The preliminary results suggest the possibility that the oral administration of Al ammonium sulfate with a dose of at least 0.075 mg/mL during the perinatal period may have effects on the development of C57BL/6J female mice. Taking into account the results of the pilot experiments, we first explored the effects of the Al compound at the dose of 0.075 mg/mL in females in Experiment 1. The compounds were dissolved in filtered tap water at a concentration of 0.075 mg/mL in Experiment 1 (defined as the low‐dose treatment) and at a concentration of 1 mg/mL in Experiment 2 (defined as the high‐dose treatment). The solution in the bottle was changed every 4‐7 days. In Experiment 1, solution intake by females was monitored every 4 days from 4 to 9 weeks of age. In Experiment 2, solution intake by males and females was measured at 20 weeks of age.
2.3. Behavioral test
In Experiment 1, female mice were subjected to a battery of behavioral tests in the following sequence: general health and neurological screen, light/dark transition, open field, elevated plus maze, hot plate, social interaction, rotarod, startle response/prepulse inhibition, Porsolt forced swim, three‐chamber social approach, T‐maze spontaneous alternation, Y‐maze, Barnes maze, tail suspension, contextual and cued fear conditioning, and home‐cage social interaction tests (see Table 1 and Appendix S1). All the mice of the vehicle‐treated and aluminum‐treated groups received the same test on the same day so that they underwent all of the behavioral tests in the same order. In Experiment 2, male and female mice underwent this battery of behavioral tests, except for the Y‐maze test, in the same manner. After each test, the floors and walls of the testing apparatuses were cleaned with 70% ethanol solution and hypochlorous acid water to prevent a bias caused by olfactory cues. The behavioral tests were performed between 9:00 am and 6:00 pm. The information about each mouse and behavioral data used in this study are available in a public database “Mouse Phenotype Database” (Available from http://www.mouse-phenotype.org/).
2.4. Light/dark transition test
The light/dark transition test, developed by Crawley and colleagues,29 was performed as previously described.30 The apparatus consisted of a cage (21 × 42 × 25 cm) divided into 2 sections of equal size by a partition with a door (O'Hara & Co., Tokyo, Japan). One chamber was brightly illuminated (390 lux), whereas the other was dark (2 lux). Mice were placed into the dark chamber and were allowed to move freely between the 2 chambers for 10 minutes with the door open. The distance travelled (cm), total number of transitions, latency to first enter the light chamber (s), and time spent in the light chamber (s) were recorded automatically using the ImageLD program (see “Image analysis”).
2.5. Elevated plus maze test
The elevated plus maze test, which has been widely used to assess anxiety,31 was performed as previously described.32 The apparatus consisted of 2 open arms (25 × 5 cm) and 2 enclosed arms of the same size with 15‐cm‐high transparent walls and a central square (5 × 5 cm) connecting the arms (O'Hara & Co.). The arms and central square were made of white plastic plates and were elevated to a height of 55 cm above the floor. To prevent mice from falling off the open arms, those arms were surrounded by a raised ledge (3 mm thick and 3 mm high). Arms of the same type were located opposite one another. Each mouse was placed in the central square of the maze facing one of the closed arms. The number of arm entries, distance travelled (cm), percentage of entries into open arms, and percentage of time spent in open arms were measured during a 10‐minute test period. Data acquisition and analysis were performed automatically using the ImageEP program.
2.6. Social interaction test
The social interaction test was conducted to measure social behavior in a novel environment, as previously described.33 Weight‐matched (within 2 g) mice of the same‐sex and same treatment group that had been housed in different cages were placed into an acrylic box together (40 × 40 × 30 cm) and allowed to explore freely for 10 minutes. The total number of contacts, total duration of contacts (s), total duration of active contacts (s), mean duration per contact (s), and total distance travelled (cm) were recorded and analyzed automatically using the ImageSI program. The active contact was measured when the 2 mice contacted each other and one or both mice moved with a velocity of at least 10 cm/s.
2.7. Three‐chamber social approach test
The three‐chamber social approach test is a well‐designed method to investigate sociability and preference for social novelty in mice.34 The apparatus consisted of a rectangular, three‐chambered box and a lid with a video camera (O'Hara & Co.). Each chamber was 20 × 40 × 47 cm, and the dividing walls were made from clear Plexiglas with a small square opening (5 × 3 cm) allowing access into each chamber. In Experiment 1, the tests were performed as previously described,34 with a slight modification as follows: subject mice were placed in the three‐chambered box and allowed to explore for 10 minutes the day before the sociability test was conducted (habituation session), and during the session, empty wire cages (9 cm in diameter, 11 cm in height, with vertical bars 0.5 cm apart) were located in the corner of each chamber. On the following day, an unfamiliar C57BL/6J female mouse (stranger 1) that had had no prior contact with the subject mice was put into the wire cage that was placed in one of the side chambers. The location of the stranger mouse in the left vs right chamber was systematically alternated between trials. The subject mouse was placed in the box and allowed to explore the entire box for a 10‐minute session to assess sociability (sociability test). Next, a second stranger female mouse was placed into the wire cage that had been empty during the first 10‐minute session to evaluate social preference for a new stranger (social novelty preference test). Thus, the subject mouse had a choice between the first, already‐investigated, now‐familiar mouse (stranger 1) and the novel unfamiliar mouse (stranger 2). In Experiment 2, the testing procedures were the same as those used in Experiment 1, except that the habituation session, sociability test, and social novelty preference tests were conducted on the same day, and male mice were used as the stranger. The amounts of time spent in each chamber and time spent around each cage were automatically calculated from video images using the ImageCSI program.
2.8. Porsolt forced swim test
The Porsolt forced swim test, developed by Porsolt et al,35 was performed to assess depression‐related behavior. Mice were placed into a Plexiglas cylinder (20 cm height × 10 cm diameter, O'Hara & Co.) filled with water (approximately 23°C) up to a height of 7.5 cm for 10 minutes per day for 2 consecutive days. The percentage of immobility time was recorded automatically using the ImagePS program.
2.9. T‐maze spontaneous alternation test
The spontaneous alternation task was conducted using a modified T‐maze apparatus and an automated video‐analyzing system,36 available through O'Hara & Co.). Mice were subjected to a spontaneous alternation session of 10 trials per day for 3 days. Each trial consists of a forced‐choice run followed by a free‐choice run. Mice were forced to enter either one of the arms for 10 seconds, and then, the door was opened so that the mouse could go back to the start compartment. They were held in the start compartment for 3 seconds and then subjected to a free‐choice run in which they were allowed to choose one of the arms. The intertrial intervals were 60 seconds. The percentage of trials in which mice entered the arm opposite to their forced‐choice run during the free‐choice run was calculated. Data acquisition and data analysis were performed using the ImageTM program.
2.10. Y‐maze test
Spatial working memory was evaluated based on spontaneous alternation behavior. The Y‐maze consists of 3 arms (labeled A, B, and C) diverging at 120 degrees from the central point (O'Hara & Co.). Each mouse was placed at the center of the maze and allowed to move freely for 10 minutes. An alternation was defined as entries into all 3 arms on consecutive occasions. For example, sequential entering into the arms in an ABCBCBCA pattern is counted as 2 alternations with the first consecutive ABC and the last consecutive BCA of 6 consecutive occasions (maximum alternation). The percentage of alternation was calculated as (alternation/maximum alternation)× 100. Data acquisition and analysis were performed automatically using the ImageYM program.
2.11. Barnes maze test
The Barnes circular maze task was conducted to test spatial reference memory37 on “dry land,” a white circular surface, 1.0 m in diameter, with 12 holes equally spaced around the perimeter (O'Hara & Co.). The circular open field was elevated 75 cm from the floor. A black Plexiglas escape box (17 × 13 × 7 cm) was located under one of the holes (target hole). The location of the target was consistent for a given mouse but randomized across mice. The maze was rotated daily, with the spatial location of the target unchanged with respect to the distal visual cues, to prevent a bias based on an olfactory cue or proximal cues. Two trials per day were conducted for 9 successive days. In each trial, the latency to first reach the target hole (s), number of errors to reach the target hole, and distance travelled to first reach the target hole (cm) were recorded by the ImageBM program. Approximately 1 day and one month after the last training, probe trials were performed without the escape box to assess spatial reference memory. In the probe tests, the time spent around each hole (s) was measured using the ImageBM program.
2.12. Tail suspension test
The tail suspension test was used to evaluate depression‐related behavior.38 Mice were suspended 30 cm above the floor in a visually isolated area by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility was recorded for a 10‐minute test period. Immobility time was measured automatically using the ImageTS program.
2.13. Contextual and cued fear conditioning test
The fear conditioning test was conducted using an automated video‐analyzing system as previously described.39 Mice were placed in a conditioning chamber (26 × 34 × 29 cm) in a sound‐attenuated room and allowed to explore freely for 2 minutes. The animals were presented with an auditory cue (55 dB white noise) that served as a conditioned stimulus (CS) for 30 seconds. During the last 2 seconds of the CS, mice were given a mild footshock (0.3 mA, 2 seconds) as an unconditioned stimulus (US). Two more CS‐US pairings were presented at 120‐second intervals. Approximately 24 hours and 1 month after the conditioning session, a context test was performed in the conditioning chamber. A cued test in an altered context was performed after the context test using a triangular box (35 × 35 × 40 cm) made of white opaque plastic, which was located in a different sound‐attenuated room. In the cued test, after the initial 3‐minute period of no CS presentation, the CS was presented during the last 3‐minute period of the test. Freezing during each minute of the test was measured automatically using the ImageFZ program in a same manner as previously described.39
2.14. Home‐cage social interaction test
The social interaction monitoring system comprised a home‐cage and a cage top with an infrared video camera (25 × 15 × 23.5 cm, interior dimensions). Two mice of the same group that had been housed separately were placed together in the home‐cage. Video images from each cage were captured at a rate of 1 frame per second. Social interaction was measured by counting the number of animals detected in each frame. We also measured the activity level of the mice by quantifying the number of pixels that changed between each pair of successive frames. Mean number of animals and total activity level in each 1‐minute bin were automatically calculated for 1 week using the ImageHA program, and the mean values averaged for each hour of the last 3 days were used for statistical analysis.
2.15. Statistical analysis
Statistical analyses were performed using SAS University Edition (SAS Institute, Cary, NC, USA). The data were analyzed to determine the effect of treatment with Al on behaviors using either an unpaired t test, a two‐way analysis of variance (ANOVA), a two‐way repeated‐measures ANOVA, or a three‐way repeated‐measures ANOVA. We defined “study‐wide significance” as statistical significance that survived the Benjamini‐Hochberg false discovery rate (FDR) correction40, 41 for controlling for multiple testing based on the number of behavioral measures of the test battery (51 measures in Experiment 1 and 50 measures in Experiment 2). “Nominal significance” was defined as having achieved statistical significance without the FDR correction (uncorrected P < .05). Post hoc multiple comparisons were further performed using Fisher's LSD as appropriate. In Experiment 2, the delivery rate of pregnant females and the survival rate from birth to weaning of pups were analyzed using Fisher's exact test. The sex ratio of litters at birth was compared by chi‐squared test. Cortical thickness and number of cells in the brain (for details, see Appendix S1) were analyzed with an unpaired t test and a two‐way ANOVA. Values in graphs are expressed as the mean ± SEM.
3. RESULTS
3.1. Experiment 1
3.1.1. Physical development, neuromuscular strength, and sensory and motor functions in low‐dose aluminum‐treated female mice
The intake of solution containing Al ammonium sulfate in Al‐treated females was approximately 3.92 mL per day from 4 weeks of age, which did not statistically differ from the volume of vehicle solution consumed by the vehicle‐treated females (Figure S1A; Treatment effect, F 1,8 = 0.02, P = .8816; Treatment × Age interaction, F 10,80 = 0.45, P = .9181). The body weights were measured every 2 weeks from 4 to 36 weeks of age. There was a significant Treatment × Age interaction on the body weights (Figure S1B; Treatment effect, F 1,35 = 0.88, P = .3551; Treatment × Age interaction, F 16,560 = 3.89, P < .0001), and the post hoc analysis indicated that there were no significant differences in the body weights at each age between Al‐ and vehicle‐treated females (for all ages, P > .05). The mean intake of Al ammonium sulfate in Al‐treated females was estimated to be approximately 16.62 mg/kg/d (0.97 mg Al/kg/d) in young adulthood (the mean body weights from 4 to 10 weeks of age were 17.77 g).
Beginning at 9 weeks of age, 20 Al‐treated females and 20 vehicle‐treated females were subjected to a battery of behavioral tests (Table 1). One Al‐treated female and one vehicle‐treated female died for unknown reasons by the onset of the test battery. The results of the statistical analyses for each test are summarized in Table S1, in which values for nominally significant effects of Treatments are highlighted in yellow. None of the statistical results of the behavioral measures reached study‐wide significance. The neurological screen indicated that Al‐treated females and vehicle‐treated females exhibited no statistically significant differences in body weight, body temperature, grip strength, or wire hang latency (Tables S1 and S2). In addition, there were no significant Treatment effects on either hot plate latency, rotarod performance, auditory startle responses to loud noise, and prepulse inhibition of the startle responses (Tables S1 and S2). These results indicate that the low‐dose oral administration of Al beginning in adolescence has no effects on physical development, pain sensitivity, or sensory and motor functions as adults in female mice.
3.1.2. Locomotor activity, anxiety‐like behavior, and depression‐related behavior in low‐dose aluminum‐treated female mice
Female mice were subjected to the open field, light/dark transition, and elevated plus maze tests, which have been extensively used to assess locomotor activity and anxiety‐like behavior. In the light/dark transition test, the data from one Al‐treated female were excluded from statistical analysis because its tail was pinched by the door of the apparatus at the beginning of the test. In the elevated plus maze test, there was a nominally significant effect of Treatment on the total arm entries (Figure 2F; Treatment effect, t 36 = 2.39, P = .0223). Al‐treated females showed more total arm entries than vehicle‐treated females. There were no significant effects of Treatment on total distance travelled, percentage of open arm entries, or percentage of time spent on open arms in the elevated plus maze (Figure 2E,G,H, and Table S1). In the open field test (Figure S2A‐D) and light/dark transition test (Figure 2A‐D), there were no significant effects of Treatment on any behavioral measures (Table S1).
Figure 2.
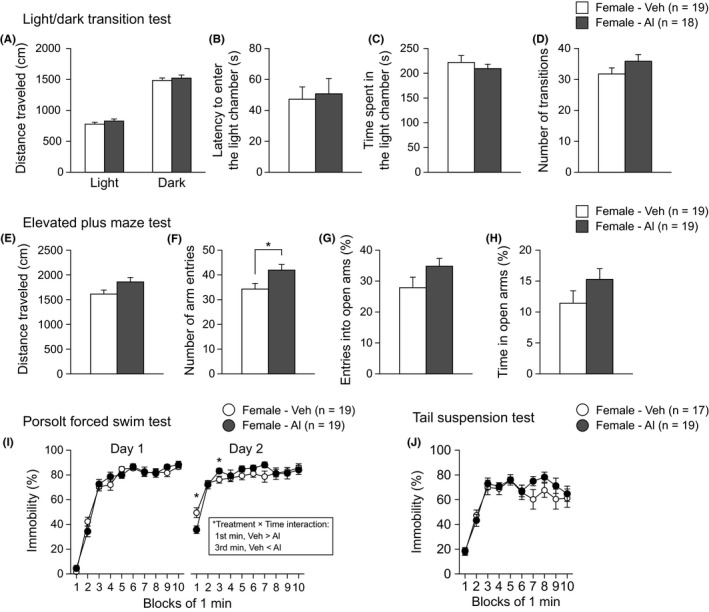
Anxiety‐like and depression‐related behaviors in low‐dose aluminum‐treated C57BL/6J females. A‐D, Light/dark transition test: (A) distance travelled (cm) in the light and dark chambers, (B) latency to enter the light chamber (s), (C) time spent in the light chamber (s), and (D) number of transitions. E‐H, Elevated plus maze test: (E) distance travelled (cm), (F) number of arm entries, (G) entries into open arms (%), and (H) time spent in open arms (%). I, Immobility time (%) in the Porsolt forced swim test. J, Immobility time (%) in the tail suspension test. Values are means ± SEM. *P < .05, which indicates a nominally statistical significance for comparisons between treatment groups
The Porsolt forced swim test and tail suspension test were performed to assess depression‐related behavior in Al‐treated mice. In the Porsolt forced swim test, there was no significant effect of Treatment on the percentage of immobility time on Day 1 (Figure 2I; Treatment effect, F 1,36 = 0.09, P = .7599; Treatment × Time interaction, F 9,324 = 1.29, P = .2436). On Day 2, there were no significant Treatment effects on immobility (F 1,36 = 0.26, P = .6108), and a significant Treatment × Time interaction on immobility was found (Figure 2I; F 9,324 = 2.02, P = .0371). The immobility time of Al‐treated females on Day 2 was significantly shorter in the first minute of the test session and significantly longer in the third minute of the session than that of vehicle‐treated females (first minute, P = .0096; third minute, P = .0492). In the tail suspension test (Figure 2J), there was no significant Treatment effect on immobility time (Table S1).
3.1.3. Social behavior in low‐dose aluminum‐treated female mice
In the social interaction test, there were no significant Treatment effects on any behavioral measures (Figure 3A‐E and Table S1). In the three‐chamber social approach test, there were no significant Treatment effects on the time spent in the chamber with a stranger female or time spent around the cage containing a stranger female (Figure 3F‐I and Table S1). Additionally, in the home‐cage social interaction test, no significant Treatment effects were found on either the activity level or the mean number of particles (Figure 3J,K, and Table S1). These results indicate normal social behavior in the low‐dose Al‐treated females.
Figure 3.
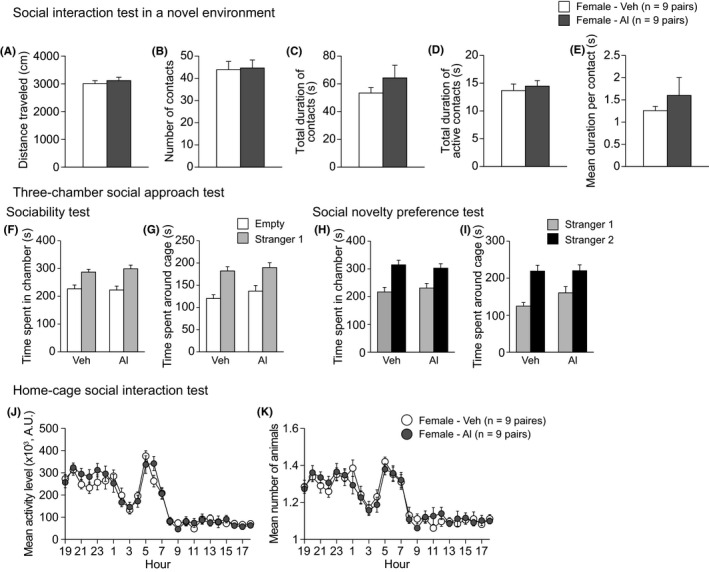
Social behavior in low‐dose aluminum‐treated C57BL/6J females. A‐E, Social interaction test: (A) distance travelled (cm), (B) number of contacts, (C) total duration of contacts (s), (D) total duration of active contacts (s), and (E) mean duration per contact (s). F‐I, Three‐chamber social approach test, which was consisted of sociability test followed by social novelty preference test: (F) time spent in the chamber with the empty cage or the cage containing a stranger mouse (stranger 1) and (G) time spent around the empty cage or the cage containing a stranger mouse (stranger 1) in the sociability test. (H) Time spent in chamber with the cage containing stranger 1 or stranger 2 and (I) time spent around cage containing stranger 1 or stranger 2 in the social novelty preference test. J, K, Home‐cage social interaction test: (J) the mean activity level and (K) mean number of animals detected over 3 d
3.1.4. Working memory, reference memory, and fear memory in low‐dose aluminum‐treated female mice
The working and reference memory of Al‐treated mice were evaluated in the T‐maze spontaneous alternation test, Y‐maze test, and Barnes maze test. In these tests, there were no significant effects of Treatment on any of the behavioral measures (Figures 4, S3A‐E, and Table S1). Contextual and cued fear memory of Al‐treated mice was assessed with the fear conditioning test. In the conditioning session (Figure 4D), there was a nominally significant effect of Treatment (F 1,35 = 10.32, P = .0028) and a significant Treatment × Time interaction on freezing (F 7,245 = 2.24, P = .0317). The post hoc analysis revealed that the freezing of Al‐treated females was significantly lower than that of vehicle‐treated females during the 4th to 6th minutes of the session (Figure 4D; P = .0328, P = .0129, and P = .0112, respectively), whereas the freezing of the 2 treatment groups did not significantly differ during the last 2 minutes of the session (Figure 4D). This result shows that Al‐treated females can acquire a conditioned fear response similar to that of vehicle‐treated females after repeated presentation of the footshock. There were no significant Treatment effects on the distance travelled during and immediately after 3 footshocks (Figure S4A), suggesting that Al exposure has no effect on pain sensitivity to footshock. There were no significant Treatment effects on freezing in the context or cued tests that were performed 1 day after conditioning (Figure 4E,F). In the context test 28 days after conditioning, significant Treatment effect was observed on freezing (F 1,35 = 6.43, P = .0158); Al‐treated females exhibited lower freezing than vehicle‐treated females (Figure 4G), while in the cued test 28 days after conditioning, there was no significant Treatment effect on freezing (Figure 4H and Table S1). These results suggest that Al‐treated females have normal working and reference memory but impaired remote contextual memory.
Figure 4.
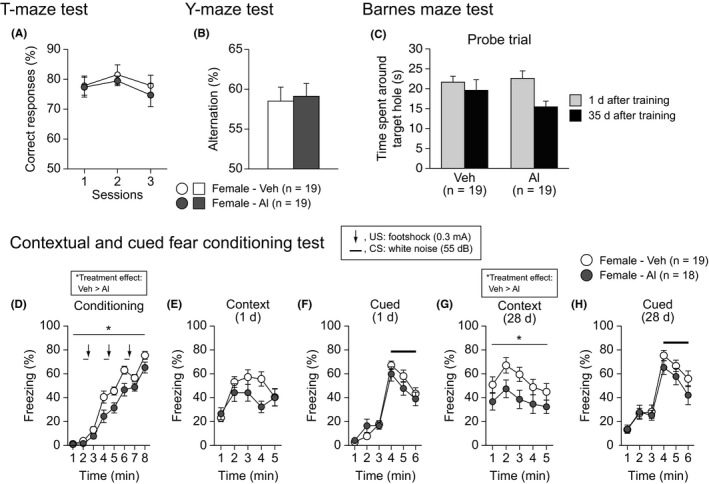
Working memory, reference memory, and fear memory in low‐dose aluminum‐treated C57BL/6J females. A, Correct responses (%) in the T‐maze test. B, Spontaneous alternation (%) in the Y‐maze test. C, Time spent around the target hole in the probe trial 1 and 35 d after the last training in the Barnes maze test. D‐H, Fear conditioning test: (D) freezing (%) in the conditioning, (E, G) context test 1 and 28 d after the conditioning, and (F, H) cued test 1 and 28 d after the conditioning. Values are means ± SEM. The asterisk indicates a nominally significance for comparisons between treatment groups (P < .05)
3.1.5. Histological features of the brain in low‐dose aluminum‐treated female mice
After the behavioral test battery, the brains from each group of mice were collected and histologically analyzed with Nissl staining. Al‐treated females exhibited no differences in morphological appearance of the brain (Figure S5), cortical thickness (Figure S5B,C; t 7 = 1.52, P = .1718), and number of cells of in the prefrontal cortex (t 7 = 1.87, P = .1034), somatosensory cortex (t 7 = 0.65, P = .5383), granule cell layer of the hippocampal dentate gyrus (t 7 = 0.13, P = .8965), and CA1 and CA3 pyramidal cell layers of the hippocampus (CA1, t 7 = 1.33, P = .2240; CA3, t 7 = 0.04, P = .9702), compared with those in vehicle‐treated females (Figure S5B,D).
3.2. Experiment 2
3.2.1. Delivery rate, litter size, sex ratio of litter, pup survival rate, and pup body weight in high‐dose aluminum‐treated mice
The delivery rate in Al‐ and vehicle‐treated pregnant females, litter size, sex ratio of the litter, pup survival rate, and pup body weights are summarized in Table 2. There were no significant differences between the treatment groups in delivery rate (P = .2105, Fisher's exact test), litter size (t 15 = 0.04, P = .9689), sex ratio of litters at birth (χ2 = .1862, P = .6661), survival rate from birth to weaning (P = .0636, Fisher's exact test), or mean pup body weights from birth to weaning (Treatment effect, F 1,27 = 2.02, P = .1669; Sex effect, F 1,27 = 2.43, P = .1308; Treatment × Sex interaction, F 1,27 = 0.05, P = .8184; Day effect, F 3,81 = 2010.90, P < .0001; Treatment × Day interaction, F 3,81 = 1.27, P = .2899; Sex × Day interaction, F 3,81 = 1.04, P = .3780; Treatment × Sex × Day interaction, F 3,81 = 0.03, P = .9943). Consequently, we could not obtain results for the effects of Al exposure on the survival of pregnant mice and the development of their pups similar to those observed in our pilot study. Although the reason for the inconsistent results is not clear, our results suggest that Al exposure at the dose used in this study has little possibility of affecting either the reproductive performance of female mice or the postnatal physical development of the pups.
Table 2.
Delivery rate, litter size, sex ratio of litter, pup survival rate, and pup body weight in high‐dose aluminum‐treated mice
| Day | Vehicle‐treated group | Aluminum‐treated group | |
|---|---|---|---|
| Number of pregnant females | 10 | 10 | |
| Delivery rate | 10/10 (100%) | 7/10 (70%) | |
| Mean litter size at birth | 7.60 ± 0.45 | 7.57 ± 0.57 | |
| Total number of pups (male) | PD0 | 40 | 25 |
| PD21 | 38 | 22 | |
| Total number of pups (female) | PD0 | 36 | 28 |
| PD21 | 36 | 25 | |
| Total number of pups (both sexes) | PD0 | 76 | 53 |
| PD21 | 74 | 47 | |
| Sex ratio at birth (male pups/total pups) | 40/76 (0.52) | 25/53 (0.47) | |
| Survival rate of pups | 74/76 (97.3%) | 47/53 (88.6%) | |
| Mean pup body weights (male) | PD0 | 1.3 ± 0.02 | 1.35 ± 0.05 |
| PD7 | 4.04 ± 0.11 | 4.19 ± 0.03 | |
| PD14 | 7.17 ± 0.21 | 7.19 ± 0.12 | |
| PD21 | 8.28 ± 0.19 | 8.65 ± 0.21 | |
| Mean pup body weights (female) | PD0 | 1.23 ± 0.02 | 1.28 ± 0.05 |
| PD7 | 3.85 ± 0.09 | 4.1 ± 0.10 | |
| PD14 | 6.96 ± 0.20 | 7.07 ± 0.12 | |
| PD21 | 7.86 ± 0.25 | 8.27 ± 0.23 |
Values in table were expressed as mean ± SEM.
The solution intakes of male and female offspring were approximately 3.58 mL/d when measured at 20 weeks of age (Figure S1C, mean solution intake: 3.79 mL/d in vehicle‐treated male; 3.60 mL/d in vehicle‐treated female; 3.37 mL/d in Al‐treated male; 3.56 mL/d in Al‐treated female; n = 5 cages, each treatment group). There was no significant Treatment effect (F 1,16 = 1.80, P = .1989) and no significant Treatment × Sex interaction (F 1,16 = 1.19, P = .2908) on the solution intake. Mean body weights measured at 19 weeks of age were 28.66 g in vehicle‐treated males (n = 20), 21.93 g in vehicle‐treated females (n = 20), 29.10 g in Al‐treated males (n = 20), and 21.64 g in Al‐treated females (n = 20). The mean oral intake of Al ammonium sulfate by Al‐treated mice was estimated to be approximately 115.80 mg/kg/d (6.88 mg Al/kg/d) for males and 164.51 mg/kg/d (9.78 mg Al/kg/d) for females in young adulthood and was approximately 10 times higher than the Al intake by females in Experiment 1.
3.2.2. Physical development, pain sensitivity, and sensory and motor functions in high‐dose aluminum‐treated mice
Twenty male and 20 female offspring from Al‐treated dams were given Al in drinking water from weaning until the end of the experiment. Similarly, 20 male and 20 female offspring from vehicle‐treated dams were given vehicle solution. After 9 weeks of age, mice were subjected to a battery of behavioral tests (Table 1). For the wire hang test, the data from one Al‐treated female were excluded from statistical analysis because the head of the mouse was caught in the wire. For the elevated plus maze test, 1 vehicle‐treated male and 2 Al‐treated males were excluded from the analysis because they fell off the apparatus during the test. The results of the statistical analyses in each test are summarized in Table S3, in which values supporting a nominally significant effect of the Treatment are highlighted in yellow. None of the statistical results of the behavioral measures reached study‐wide significance.
Al‐treated male and female mice appeared normal, and their righting, whisker twitch, and ear twitch reflexes were intact. There were no significant main effects of Treatment on the body weights, body temperature, grip strength, wire hang latency, hot plate latency, or rotarod latency in either sex (Tables S2 and S3). These data indicate that long‐term treatment with Al has no effects on physical development from weaning to adulthood and on neuromuscular strength, pain sensitivity, or motor function in adulthood in male and female mice.
There were statistically significant main effects of Sex on body weight (F 1,76 = 463.01, P < .0001), grip strength (F 1,76 = 21.32, P < .0001), wire hang latency (F 1,75 = 18.89, P < .0001), hot plate latency (F 1,76 = 4.91, P = .0298), and rotarod latency (F 1,76 = 15.47, P = .0002), but not on body temperature (Tables S2 and S3). The results showed that male mice had heavier bodies, greater grip strength, shorter wire hang latency, longer hot plate latency, and shorter rotarod latency than female mice.
There was no significant main effect of Treatment on the startle responses to 110‐ or 120‐dB stimulus (Tables S2 and S3). A significant Treatment × Trial interaction on the startle responses was found (F 1,75 = 5.56, P = .0210), but the post hoc analysis revealed that the results did not reach statistical significance at each startle stimulus. A nominally significant effect of Treatment was found on the prepulse inhibition (F 1,75 = 4.12, P = .0460), and Al‐treated mice exhibited lower prepulse inhibition than vehicle‐treated mice. In those tests, there were neither significant effects of Sex nor a significant Treatment × Sex interaction (Table S3). These data suggest that the high‐dose, long‐term administration of Al induces a decreased sensorimotor gating function in male and female mice.
3.2.3. Locomotor activity, anxiety‐like behavior, and depression‐related behavior in high‐dose aluminum‐treated mice
No significant main effects of Treatment were observed on any of the behavioral measures in the open field, light/dark transition, elevated plus maze, Porsolt forced swim, and tail suspension tests (Figures 5, S2E‐H, and Table S3). These results indicate that long‐term exposure to a relatively high dose of Al has no effects on locomotor activity, anxiety‐like behavior, or depression‐related behavior under the different testing conditions in male and female mice.
Figure 5.
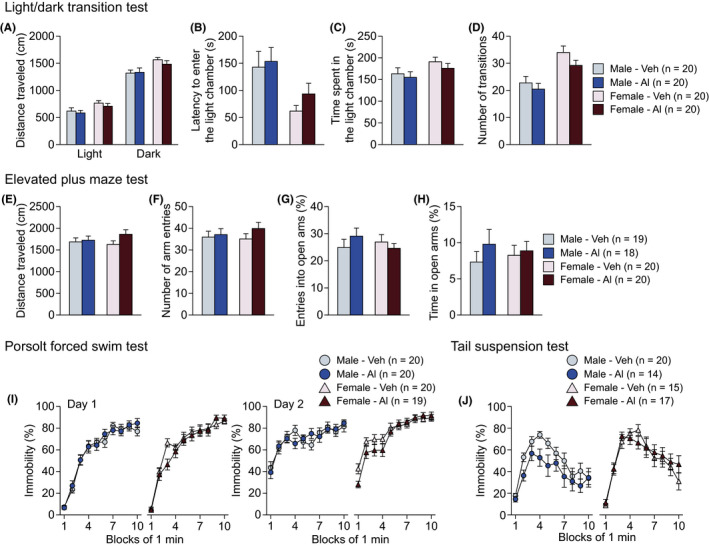
Anxiety‐like and depression‐like behaviors in high‐dose aluminum‐treated C57BL/6J mice. A‐D, Light/dark transition test: (A) distance travelled (cm) in the light and dark chambers, (B) latency to enter the light chamber (s), (C) time spent in the light chamber (s), and (D) number of transitions. E‐H, Elevated plus maze test: (E) distance travelled (cm), (F) number of arm entries, (G) entries into open arms (%), and (H) time spent in open arms (%). I, Immobility time (%) on days 1 and 2 in the Porsolt forced swim test. J, Immobility time (%) in the tail suspension test. Values are means ± SEM
In the open field test, there were significant main effects of Sex on distance travelled (Figure S2E; F 1,76 = 6.30, P = .0142) and vertical activity (Figure S2F; F 1,76 = 7.63, P = .0072). Male mice travelled a shorter distance but showed more vertical activity than female mice in the open field. In the light/dark transition test, there were also significant main effects of Sex on distance travelled in the light and dark chambers (Figure 5A; light chamber, F 1,76 = 6.72, P = .0114; dark chamber, F 1,76 = 10.01, P = .0022), latency to enter the light chamber (Figure 5B; F 1,76 = 9.97, P = .0023), time spent in the light chamber (Figure 5C; F 1,76 = 3.99, P = .0492), and number of transitions (Figure 5D; F 1,76 = 20.15, P < .0001). Male mice exhibited shorter distance travelled, longer latency to enter the light chamber, shorter time spent in the light chamber, and a lower number of transitions than female mice in the light/dark transition test. There were no significant effects of Sex on the behavioral measures in the elevated plus maze test. Overall, these results indicate the possibility that, compared with female mice, male mice show decreased locomotor activity and increased anxiety‐like behavior in novel environments, whereas the treatment with high‐dose Al had no effects on those behaviors in male and female mice.
In the Porsolt forced swim test, there were significant Sex × Time interactions in immobility on Day 2 (Figure 5I; F 9,675 = 4.15, P < .0001). Females’ immobility time was longer than that of the males in the second half of the period on Day 2 (5 minutes, P = .0304; 6 minutes, P = .0011; 7 minutes, P = .0119; 8 minutes, P = .0143; 9 minutes, P = .0012). Similarly, in the tail suspension test, there was a significant main effect of Sex and a significant Sex × Time interaction on immobility (Figure 5J; F 1,62 = 5.13, P = .0270; F 9,558 = 2.09, P = .0286, respectively). Post hoc analysis revealed that females showed a lower immobility time than males at the first minute of the test (P = .0163), and then, females exhibited a higher immobility time than males at the 5th and 8th minutes of the test (5 minutes, P = .0108; 8 minutes, P = .0148). Collectively, these data indicate that female mice are more immobile during the second half of the period than are male mice regardless of the administration of Al.
3.2.4. Social behavior in high‐dose aluminum‐treated mice
No significant main effects of Treatment were found on any of behavioral measures in the social interaction test, the three‐chamber social approach test, or the home‐cage social interaction test (Figure 6 and Table S3). In the social interaction test, there were significant main effects of Sex on distance travelled (Figure 6A; F 1,36 = 7.41, P = .0099), total duration of contacts (Figure 6C; F 1,36 = 6.74, P = .0135), and mean duration per contact (Figure 6E; F 1,36 = 7.30, P = .0104). Male mice travelled a shorter distance and exhibited a longer duration of contacts than female mice. In the three‐chamber social approach test, there were no significant main effects of Sex on the behavioral measures (Figure 6F‐I and Table S3). In the home‐cage social interaction test, significant main effects of Sex were found on activity level (Figure 6J; F 1,23 = 9.24, P = .0058) and mean number of particles (Figure 6K; F 1,23 = 8.44, P = .0080). Male mice exhibited a lower activity level and a smaller mean number of particles than female mice in their home cage. In the social interaction test, there were significant Treatment × Sex interactions in the total duration of active contacts (Figure 6D; F 1,36 = 4.73, P = .0364) and the mean duration per contact (Figure 6E; F 1,36 = 5.49, P = .0248). The post hoc analysis showed that Al‐treated females exhibited more active contacts than vehicle‐treated females (P = .0236), but there were no statistically significant differences in the mean duration per contact between the treatment groups in each sex. Overall, these results suggest that male mice show lower locomotor activity and higher social interaction than female mice in both novel environments and the home cage, whereas in females, the oral administration of Al induces an increase in active social contact in novel environments.
Figure 6.
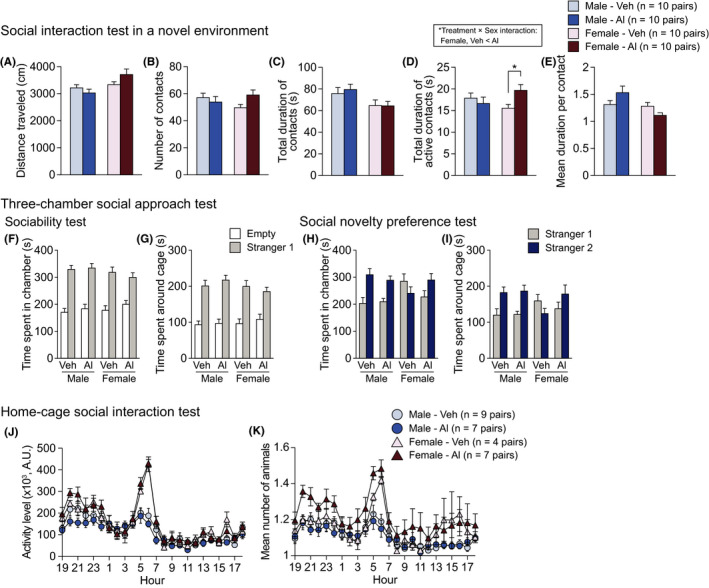
Social behavior in high‐dose aluminum‐treated C57BL/6J mice. A‐E, Social interaction test: (A) distance travelled (cm), (B) number of contacts, (C) total duration of contacts (s), (D) total duration of active contacts (s), and (E) mean duration per contact (s). F‐I, Three‐chamber social approach test, which was consisted of sociability test followed by social novelty preference test: (F) time spent in the chamber with the empty cage or the cage containing a stranger mouse (stranger 1) and (G) time spent around the empty cage or the cage containing a stranger mouse (stranger 1) in the sociability test. (H) Time spent in chamber with the cage containing stranger 1 or stranger 2 and (I) time spent around cage containing stranger 1 or stranger 2 in the social novelty preference test. J, K, Home‐cage social interaction test: (J) the mean activity level and (K) mean number of animals detected over 3 d. Values are means ± SEM. The asterisk indicates a nominally significance for comparisons between treatment groups (P < .05)
3.2.5. Working memory, reference memory, and fear memory in high‐dose aluminum‐treated mice
Working memory was assessed with the T‐maze spontaneous alternation task. There was a nominally significant effect of Treatment on the percentage of correct responses (Figure 7A; F 1,73 = 5.48, P = .0220). There was no significant main effect of Sex, no significant Treatment × Sex interaction, no significant Treatment × Session interaction, and no significant Treatment × Sex × Session interaction on the percentage of spontaneous alternation (Table S3). The data indicated that Al‐treated mice exhibited a lower percentage of spontaneous alternation than vehicle‐treated mice, suggesting that the high‐dose, long‐term administration of Al induces working memory deficits regardless of sex.
Figure 7.
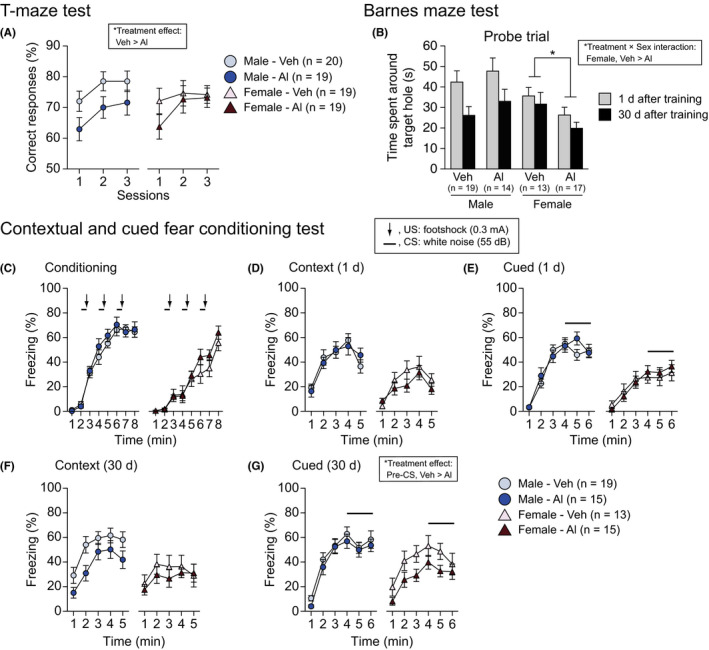
Working memory, reference memory, and fear memory in high‐dose aluminum‐treated C57BL/6J mice. A, Spontaneous alternation (%) in the T‐maze test. B, Time spent around the target hole in the probe test 1 and 30 d after the last training in the Barnes maze test. C‐G, Fear conditioning test: (C) freezing (%) in the conditioning, (D, F) context test 1 and 30 d after the conditioning, and (E, G) cued test 1 and 30 d after the conditioning. Values are means ± SEM. The asterisk indicates a nominally significance for comparisons between treatment groups (P < .05)
Spatial reference memory was evaluated using the Barnes maze test (Figures 7B and S3F‐J). In the training session, there was a nominally significant Treatment effect and a significant Treatment× Sex × Block interaction on the number of errors to reach the target hole (Figure S3G; F 1,59 = 4.84, P = .0317; F 9,531 = 2.57, P = .0067). The post hoc analysis revealed that Al‐treated female mice exhibited a greater number of errors than vehicle‐treated female mice in the first block of the session (P = .0378). There was also a significant effect of Treatment and a significant Treatment × Sex × Block interaction on the distance travelled to reach the target hole (Figure S3H; F 1,59= 4.53, P = .0375; F 9,531 = 2.10, P = .0283). The post hoc analysis showed that in the sixth block of the session, Al‐treated male mice travelled further than vehicle‐treated male mice (P = .0069). In the last block of training session, no significant differences between the treatment groups were found in the number of errors and distance travelled, indicating that Al‐treated mice exhibited a reference memory performance similar to that of vehicle‐treated mice after training. In the probe trials, there was no significant main effect of Treatment, no significant Treatment × Trial interaction, and no significant Treatment × Sex × Trial interaction on the time spent around the target hole (Figures 7B, S3I,J, and Table S3). There was a significant Treatment × Sex interaction on the time spent around the target hole (Figure 7B; F 1,59 = 4.79, P = .0326). The post hoc analysis revealed that Al‐treated females spent a shorter time around the target hole than vehicle‐treated females in the probe trials (P = .0213), indicating that long‐term treatment with Al induces spatial memory deficits in female mice.
In the conditioning session of the fear conditioning test, there were no significant effects of Treatment, no significant Treatment × Sex interaction, no significant Treatment × Time interaction, and no significant Treatment × Sex × Time interaction on freezing during the conditioning (Figure 7C and Table S3), or distances travelled during and after footshock (Figure S4B and Table S3), except for a significant Treatment × Sex × Time interaction on distance travelled during and after the second footshock (F 14,812 = 1.74, P = .0439). Overall, these results indicate no apparent treatment effects on behavioral responses during the conditioning session.
In the context and cued tests 1 day after the conditioning, there was no significant main effect of Treatment, no significant Treatment × Sex interaction, no significant Treatment × Time interaction, and no significant Treatment × Sex × Time interaction on freezing (Figure 7D,E, and Table S3). Similarly, in the context test 30 days after conditioning, there were no significant main effect of Treatment and no significant interactions on freezing (Figure 7F and Table S3). In the cued test 30 days after the conditioning, there were nominally significant effects of Treatment on freezing (Figure 7G; F 1,58 = 4.63, P = .0356) during the pre‐CS period. The data showed that Al‐treated mice exhibited slightly less freezing than vehicle‐treated mice during the pre‐CS period, suggesting a reduced generalized fear response 30 days after the aversive experience in Al‐treated mice.
There were significant main effects of Sex on freezing for the conditioning (Figure 7C; F 1,58 = 47.55, P < .0001), the context test 1 day after the conditioning (Figure 7D; F 1,58 = 27.29, P < .0001), the pre‐CS period (Figure 7E; F 1,58 = 10.92, P = .0016), and the CS period (Figure 7E; F 1,58 = 28.76, P < .0001) of the cued test 1 day after the conditioning, the context test 30 days after the conditioning (Figure 7F; F 1,58 = 7.72, P = .0074), and the CS period of the cued test 30 days after the conditioning (Figure 7G; F 1,58 = 8.23, P = .0057). Overall, males displayed significantly more freezing than female mice in each test session.
3.2.6. Histological features of the brain in high‐dose aluminum‐treated female mice
The brains from each group of mice were histologically analyzed with Nissl staining. There were no differences in morphological appearance of the prefrontal cortex and hippocampus, cortical thickness (F 1,12 = 1.94, P = .1890), and number of cells in the prefrontal cortex (F 1,12 = 1.60, P = .2305), sensorimotor cortex (F 1,12 = 0.22, P = .6505), hippocampal dentate gyrus (F 1,12 = 0.15, P = .7059), and CA1 (F 1,12 = 0.26, P = .6175) and CA3 (F 1,12 = 0.10, P = .7582) of the hippocampus, compared with those in vehicle‐treated females (Figure S5A,E,F).
4. DISCUSSION
Al ammonium sulfate is an Al compound that is widely used as a food additive. However, the behavioral effects resulting from the oral intake of Al ammonium sulfate remain to be examined. In this study, we investigated the effects of the long‐term oral administration of Al ammonium sulfate in drinking water on various types of behavior in adult C57BL/6J mice using a battery of behavioral tests. The results of Experiment 1, although not reaching study‐wide significance, showed that the administration of Al ammonium sulfate in drinking water from 4 weeks of age at a relatively low dose (estimated to be 0.97 mg Al/kg/d) might induce an increased number of arm entries in the elevated plus maze test, an initial decrease and subsequent increase in immobility in the Porsolt forced swim test, and decreased freezing in the same context as conditioning 1 month after the conditioning session in female C57BL/6J mice as adults. However, those behavioral differences between Al‐ and vehicle‐treated females of the low‐dose experiment were not found in the high‐dose condition of Experiment 2 (the doses were estimated to be 6.88 mg Al/kg/d in adult males and 9.78 mg Al/kg/d in adult females). One of the possible explanations for the inconsistent results is differences in age of animals subjected to the behavioral tests between Experiments 1 and 2. In Experiment 2, the mice were tested at 7 months old in the Porsolt forced swim test and at 21 to 23 months old in the fear conditioning test, while in Experiment 1, the ages of mice were 3 months in the Porsolt forced swim test and 7 months in the fear conditioning test. Consistent with our previous report showing age‐related changes in behaviors,42 in this study, immobility and freezing were lower in older mice in Experiment 2 than in younger mice in Experiment 1. Thus, no differences in the behaviors between aluminum‐ and vehicle‐treated mice in Experiment 2 might be potentially due to a floor effect. In the elevated plus maze test, in which mice were tested at 2‐3 months of age in both Experiments 1 and 2, the total number of arm entries was higher in aluminum‐treated females than vehicle‐treated females in Experiment 2 as seen in Experiment 1, although the results did not reach the significance level. The similar but slight changes in the total arm entries found in Experiments 1 and 2 suggest another possibility that the 2 doses used in this study might not be within the range of doses that are sufficient to induce marked behavioral changes. Therefore, oral exposure to low‐dose aluminum might yield statistically false‐positive results for some behavioral differences found in Experiment 1. The results of the high‐dose experiment showed that Al exposure from the prenatal period at a high dose can cause an increased duration of active social contacts and reduced reference memory performance only in females and decreased PPI, decreased spontaneous alternation, and slight increase in freezing to altered context in C57BL/6J mice, although the differences in any of the behavioral measures assessed between Al‐ and vehicle‐treated mice did not reach a study‐wide significance level. These findings suggest that, while long‐term oral exposure of Al ammonium sulfate may have a potential effect on inducing behavioral changes in C57BL/6J mice in a sex‐dependent manner, the behavioral effects of Al exposure at the doses used in this study, if any, seem small.
Numerous rodent studies have shown that Al exposure during the perinatal period and/or adulthood has deleterious effects on physiological and behavioral development, including loss of body weight, decreased locomotor activity, impaired active avoidance, and decreased spatial memory performance.12, 15, 16, 18, 19, 20, 21, 24, 43, 44, 45 The doses of Al orally administered to animals in most previous studies seem to be relatively high and are estimated to be a few to more than 10 times higher than the doses given to the mice (0.97‐9.78 mg Al/kg/d) in this study. Considering the potential effects of Al exposure found in this study, it is suggested that long‐term exposure to Al at relatively low doses can lead to slight behavioral changes, especially memory performance. However, in this study, Al exposure did not significantly affect physical development, motor performance, anxiety‐like behavior, and social behavior. The lack of Al effects on those behaviors might be explained by the relatively short period of Al exposure. In humans, dietary intake was estimated to be 0.2‐2.3 mg Al/kg/wk at an assumed body weight of 60 kg, which is generally within the provisional tolerable weekly intake (PTWI) of 2 mg Al/kg/wk.1 The PTWI of 2 mg/kg/wk (0.28 mg/kg/d) was established based on a rat study showing no observable adverse effect level (NOAEL) of 30 mg Al/kg/d, dividing the NOAEL by an uncertainty factor of 100, in addition to taking into consideration the lowest observed adverse effect level (LOAEL) range of 50‐75 mg/kg/d in animal studies.1 The potential behavioral effects of Al exposure at lower doses in this study suggest that further study will be needed to replicate our behavioral results and reevaluate the effects of Al on brain functions in detail. Additionally, because in vivo pharmacokinetics of aluminum compounds including aluminum ammonium sulfate remain to be largely unknown, it is important to determine the pharmacokinetics of each aluminum compound in order to understand the mechanisms underlying the behavioral changes caused by exposure to Al compounds at different doses. Although the present study measured oral Al intake of mice to estimate the dose of Al, absorption and excretion of Al would need to be monitored by measurement of Al concentrations in organs, blood, and urine.
Al injected subcutaneously into pregnant rats can be transferred to the fetuses at 0.2% Al and is detected in the brain and peripheral organs of the fetuses.46, 47 Similarly, Al has been reported to be found in the brains of suckling pups after daily subcutaneous injections of Al to lactating rats.46, 47 The oral intake of dietary Al is absorbed into systemic circulation at only <0.3% of intake,48 and Al can be broadly distributed throughout the brain and accumulate in all brain regions, including the cortex, hippocampus, striatum, and cerebellum in rodents,49, 50, 51 potentially having various effects on brain functions. In rats, exposure to Al chloride (100 mg/kg body weight) for 8 weeks induced an increase in lipid peroxidation and decreases in superoxide dismutase and catalase activity in the brain, suggesting that Al has pro‐oxidant and neurotoxic effects on the brain.52 Furthermore, Al exposure with different doses and different routes of administration increased the expression of glial fibrillary acidic protein, a marker of astrocyte, and reactive microglia in the rodent brain,13, 53, 54, 55 suggesting an Al‐induced increase in brain inflammation. Indeed, the oral intake of either Al lactate in drinking water (0.01‐1 mmol/L) for 10 weeks or Al ammonium sulfate in drinking water (5‐125 ppm) for 4 weeks resulted in increases in inflammatory cytokines such as nuclear factor‐κB (NF‐κb), tumor necrosis factor‐α (TNF‐α), and interleukin‐1β (IL‐1β) in the mouse brain.53, 56 Several lines of evidence indicate that brain inflammation is associated with alterations in brain functions, including neurotransmitter metabolism, neuroendocrine function, synaptic plasticity, and neurocircuits, leading to neuropsychiatric and neurological diseases.57, 58, 59, 60 It has been suggested that intake of 0.3% Al chloride in drinking water from birth induces the impairment of synaptic connectivity in the mouse hippocampus.61 Daily oral intake of Al chloride (300‐600 mg/kg body weight) has also been reported to result in decreases in dopamine and 5‐HT levels in rat and mouse brains,12, 62 and intraperitoneal injection with Al (4.2 mg/kg body weight, as Al chloride hexahydrate) for 4 weeks increased the level of glutamate and decreased the level of GABA in the brain.63, 64 In this study, the histological features of the prefrontal cortex, somatosensory cortex, and hippocampus appeared to be normal in Al‐treated mice, possibly supporting the findings that the long‐term oral administration of Al ammonium sulfate at the low doses has no marked effect on behaviors. However, the exposure to the Al compound might cause chronic brain inflammation and alternations in neurotransmission systems that may contribute to the slight behavioral changes observed in this study.
Sex differences were observed for almost all of the behaviors assessed without considering the effect of Al treatment. Although male mice had greater grip strength than female mice, the latency to fall off the wire in the wire hang test and the latency to fall off the rotating rod in the rotarod test were lower in males than in females. Previous studies have reported a negative correlation between body weight and rotarod performance.42, 65, 66 Therefore, males’ lower rotarod performance might be attributable to their heavier body weights. The results of the hot plate test suggest lower pain sensitivity in males, in agreement with Mogil et al's67 finding that male mice were less sensitive than female mice in some nociceptive tests. Sex differences in locomotor activity and anxiety‐like behavior have been reported to be dependent on strain, apparatus, and measure used.68, 69, 70 In our study, male mice showed lower locomotor activity than female mice in several tests, including the open field test, the light/dark transition test, the social interaction test, and the fear conditioning test. These results suggest that females may be less anxious than males under our testing conditions in the C57BL/6J strain of mice.
The higher general activity in females might affect the results for sex differences in the other behavioral measures. For example, the hyperactive phenotype might have resulted in the shorter latency to enter the light chamber, increased number of transitions, and increased time spent in the light chamber in the light/dark transition test, along with the decreases in the total duration of contacts and mean duration per contact in the social interaction test and decreased immobility in the fear conditioning test. In the Porsolt forced swim test, although females showed an increased distance travelled compared with that of males during the early test period, females eventually exhibited more immobility and shorter distance travelled during the late period than males. A similar result for the sex difference was observed in the tail suspension test. Although the sex difference in depression‐related behavior remains controversial (for review, see71), these results suggest that female mice show depression‐related behavior in response to a stressful environment. This outcome appears to resemble human depression, which women are approximately twice as likely to suffer than men.72, 73 This study also found a sex difference in that males exhibited better performance in the spatial memory task than females, which is consistent with previous findings.74, 75, 76, 77 To our knowledge, there is little literature reporting sex differences in the behavioral effects of Al exposure. We found sex‐dependent effects of Al exposure on some behavioral measures. Al exposure induced increased active social contacts in the social interaction test and decreased time spent around the target hole in the Barnes maze test in females, but not in males. The sex differences in behavior might be attributable to a difference in Al intake between the sexes. Indeed, the daily Al intake per body weight was higher in females than in males, potentially explaining the sensitivity of the behavioral effects of Al exposure in females. Even then, because of the lack of information regarding the sex‐dependent effects of the oral intake of Al ammonium sulfate, further study will be needed to obtain a better understanding of the mechanisms of the behavioral effects of Al in both sexes.
Some populations, particularly children, are estimated to regularly consume Al‐containing foods such as cakes, cookies, and snacks; therefore, the provisional tolerable weekly intake of Al is likely to be exceeded to a large extent.1 Dietary excessive intake of Al early in life may be a potential risk factor for brain dysfunction. In daily life, exposure to Al compounds can occur via sources other than foods, for example, through medical treatment. Al compounds are widely used in medicines and vaccines, such as antacids, analgesics, the influenza vaccine, and the human papillomavirus vaccine. Al chloride and Al hydroxide are used as adjuvants in vaccines for the prevention and treatment of people infected with viruses. In mice, it has been reported that 6 times subcutaneous injections of Al hydroxide used in vaccines during 2‐3 weeks, even at a low dose (total 0.1 or 0.55 mg Al/kg, corresponding to the clinical doses), induced decreased locomotor activity, increased anxiety‐like behavior, and induced a spatial memory deficit.78, 79 However, there seems to be little possibility that such a short‐term and low‐dose Al exposure produces substantial behavioral changes, as suggested by the findings of this study, although there were differences in the form of the Al, the administration route, the exposure duration, and the behavioral testing between the studies. These findings indicate the necessity of further investigations on the effects of long‐term Al exposure from early life at relatively low doses in future animal and human studies.
5. CONCLUSIONS
This study failed to detect any changes exceeding a study‐wide significance level in behaviors of C57BL/6J mice by long‐term oral exposure of Al ammonium sulfate, while the possibility that it may lead to slight behavioral changes, such as altered immobility in forced swim test, decreased prepulse inhibition, and impaired memory performances, cannot be excluded. Additional research is required to further evaluate whether reliable, reproducible results for the behavioral effects of the oral exposure of Al ammonium sulfate can be obtained and to determine the precise brain mechanism(s) underlying the effects of Al, if any.
CONFLICT OF INTEREST
The authors declare no conflict of interest for this article. [Correction added on 5 March 2018, after first online publication: The words, ‘for this article’, have been added to the back of the conflict of interest statement.]
DATA REPOSITORY
All data are available in the Supporting Information data file.
ANIMAL STUDIES
All of the experimental procedures were approved by the Institutional Animal Care and Use Committee of Kobe University (# P110604‐R1) and of Fujita Health University (#I0741).
Supporting information
ACKNOWLEDGMENTS
We thank the members of our laboratory for their support, especially S. Yoshikawa, T. Yokoi, and T. Murakami for animal husbandry and behavioral experiment. This study was supported by Grants‐in‐Aid for research on Food Safety from the Food Safety commission of Japan and for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan. Behavioral analysis was performed at the Institute for Comprehensive Medical Science, Fujita Health University (Joint Usage Research Center for Genes, Brain and Behavior accredited by MEXT) in Japan.
Shoji H, Irino Y, Yoshida M, Miyakawa T. Behavioral effects of long‐term oral administration of aluminum ammonium sulfate in male and female C57BL/6J mice. Neuropsychopharmacol Rep. 2018;38:18–36. 10.1002/npr2.12002
REFERENCES
- 1. Joint FAO/WHO Expert Committee on Food Additives . Evaluation of certain food additives and contaminants: seventy‐fourth report. Volume 65. Geneva: WHO Press; 2011. [Google Scholar]
- 2. Exley C. A molecular mechanism of aluminium‐induced Alzheimer's disease? J Inorg Biochem. 1999;76:133–40. [DOI] [PubMed] [Google Scholar]
- 3. Grant WB, Campbell A, Itzhaki RF, Savory J. The significance of environmental factors in the etiology of Alzheimer's disease. J Alzheimer's Dis. 2002;4:179–89. [DOI] [PubMed] [Google Scholar]
- 4. Polizzi S, Pira E, Ferrara M, et al. Neurotoxic effects of aluminium among foundry workers and Alzheimer's disease. Neurotoxicology. 2002;23:761–74. [DOI] [PubMed] [Google Scholar]
- 5. Perl DP, Gajdusek DC, Garruto RM, Yanagihara RT, Gibbs CJ. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and Parkinsonism‐dementia of Guam. Science. 1982;217:1053–5. [DOI] [PubMed] [Google Scholar]
- 6. Perl DP, Moalem S. Aluminum, Alzheimer's disease and the geospatial occurrence of similar disorders. Rev Mineral Geochem. 2006;64:115–34. [Google Scholar]
- 7. Garruto RM, Fukatsu R, Yanagihara R, Gajdusek DC, Hook G, Fiori CE. Imaging of calcium and aluminum in neurofibrillary tangle‐bearing neurons in parkinsonism‐dementia of Guam. Proc Natl Acad Sci. 1984;81:1875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirsch EC, Brandel J‐P, Galle P, Javoy‐Agid F, Agid Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson's disease: an X‐ray microanalysis. J Neurochem. 1991;56:446–51. [DOI] [PubMed] [Google Scholar]
- 9. Kawahara M, Kato‐Negishi M. Link between aluminum and the pathogenesis of Alzheimer's disease: the integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimer's Dis. 2011;2011:e276393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lidsky TI. Is the aluminum hypothesis dead? J Occup Environ Med. 2014;56:S73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomljenovic L. Aluminum and Alzheimer's disease: after a century of controversy, is there a plausible link? J Alzheimer's Dis. 2011;23:567–98. [DOI] [PubMed] [Google Scholar]
- 12. Abu‐Taweel GM, Ajarem JS, Ahmad M. Neurobehavioral toxic effects of perinatal oral exposure to aluminum on the developmental motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol Biochem Behav. 2012;101:49–56. [DOI] [PubMed] [Google Scholar]
- 13. Erazi H, Sansar W, Ahboucha S, Gamrani H. Aluminum affects glial system and behavior of rats. CR Biol. 2010;333:23–7. [DOI] [PubMed] [Google Scholar]
- 14. Golub MS, Han B, Keen CL, Gershwin ME, Tarara RP. Behavioral performance of Swiss Webster mice exposed to excess dietary aluminum during development or during development and as adults. Toxicol Appl Pharmacol. 1995;133:64–72. [DOI] [PubMed] [Google Scholar]
- 15. Gonda Z, Lehotzky K, Miklósi A. Neurotoxicity induced by prenatal aluminum exposure in rats. Neurotoxicology. 1995;17:459–69. [PubMed] [Google Scholar]
- 16. Gonda Z, Lehotzky K. Effect of prenatal aluminium lactate exposure on conditioned taste aversion and passive avoidance task in the rat. J Appl Toxicol. 1996;16:529–32. [DOI] [PubMed] [Google Scholar]
- 17. Julka D, Sandhir R, Gill KD. Altered cholinergic metabolism in rat CNS following aluminum exposure: implications on learning performance. J Neurochem. 1995;65:2157–64. [DOI] [PubMed] [Google Scholar]
- 18. Kumar A, Dogra S, Prakash A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: possible behavioral and biochemical alterations in rats. Behav Brain Res. 2009;205:384–90. [DOI] [PubMed] [Google Scholar]
- 19. Santucci D, Rankin J, Laviola G, Aloe L, Alleva E. Early exposure to aluminium affects eight‐arm maze performance and hippocampal nerve growth factor levels in adult mice. Neurosci Lett. 1994;166:89–92. [DOI] [PubMed] [Google Scholar]
- 20. Colomina MT, Roig JL, Sánchez DJ, Domingo JL. Influence of age on aluminum‐induced neurobehavioral effects and morphological changes in rat brain. Neurotoxicology. 2002;23:775–81. [DOI] [PubMed] [Google Scholar]
- 21. Colomina MT, Sanchez DJ, Domingo JL, Sanchez‐Turet M. Exposure of pregnant mice to aluminum and restraint stress: effects on postnatal development and behavior of the offspring. Psychobiology. 1999;27:521–9. [Google Scholar]
- 22. Connor DJ, Jope RS, Harrell LE. Chronic, oral aluminum administration to rats: cognition and cholinergic parameters. Pharmacol Biochem Behav. 1988;31:467–74. [DOI] [PubMed] [Google Scholar]
- 23. Doming JL, Llorens J, Sanchez DJ, Gomez M, Llobet JM, Corbella J. Age‐related effects of aluminum ingestion on brain aluminum accumulation and behavior in rats. Life Sci. 1996;58:1387–95. [DOI] [PubMed] [Google Scholar]
- 24. Roig JL, Fuentes S, Colomina MT, Vicens P, Domingo JL. Aluminum, restraint stress and aging: behavioral effects in rats after 1 and 2 years of aluminum exposure. Toxicology. 2006;218:112–24. [DOI] [PubMed] [Google Scholar]
- 25. Thorne BM, Cook A, Donohoe T, Lyon S, Medeiros DM, Moutzoukis C. Aluminum toxicity and behavior in the weanling Long‐Evans rat. Bull Psychon Soc. 1987;25:129–32. [Google Scholar]
- 26. Hirata‐Koizumi M, Fujii S, Ono A, et al. Evaluation of the reproductive and developmental toxicity of aluminium ammonium sulfate in a two‐generation study in rats. Food Chem Toxicol. 2011;49:1948–59. [DOI] [PubMed] [Google Scholar]
- 27. Sato K, Suzuki I, Kubota H, et al. Estimation of daily aluminum intake in Japan based on food consumption inspection results: impact of food additives. Food Sci Nutr. 2014;2:389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yokel RA. Food additive In: El‐Samragy Y, editor. Aluminium in food – the nature and contribution of food additives. Rijeka: Intech, 2012; p. 203–228. [Google Scholar]
- 29. Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–70. [DOI] [PubMed] [Google Scholar]
- 30. Takao K, Miyakawa T. Light/dark transition test for mice. J Vis Exp. 2006;1:e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lister RG. The use of a plus‐maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–5. [DOI] [PubMed] [Google Scholar]
- 32. Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp. 2008;22:e1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuda I, Shoji H, Yamasaki N, Miyakawa T, Aiba A. Comprehensive behavioral phenotyping of a new Semaphorin 3 F mutant mouse. Mol Brain. 2016;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moy SS, Nadler JJ, Perez A, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic‐like behavior in mice. Genes Brain Behav. 2004;3:287–302. [DOI] [PubMed] [Google Scholar]
- 35. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. [DOI] [PubMed] [Google Scholar]
- 36. Shoji H, Hagihara H, Takao K, Hattori S, Miyakawa T. T‐maze forced alternation and left‐right discrimination tasks for assessing working and reference memory in mice. J Vis Exp. 2012;60:e3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. [DOI] [PubMed] [Google Scholar]
- 38. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–70. [DOI] [PubMed] [Google Scholar]
- 39. Shoji H, Takao K, Hattori S, Miyakawa T. Contextual and cued fear conditioning test using a video analyzing system in mice. J Vis Exp. 2014;85:e50871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–84. [DOI] [PubMed] [Google Scholar]
- 41. Kafkafi N, Lipkind D, Benjamini Y, Mayo CL, Elmer GI, Golani I. SEE locomotor behavior test discriminates C57BL/6J and DBA/2J mouse inbred strains across laboratories and protocol conditions. Behav Neurosci. 2003;117:464–76. [DOI] [PubMed] [Google Scholar]
- 42. Shoji H, Takao K, Hattori S, Miyakawa T. Age‐related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain. 2016;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cherroret G, Bernuzzi V, Desor D, Hutin M‐F, Burnel D, Lehr PR. Effects of postnatal aluminum exposure on choline acetyltransferase activity and learning abilities in the rat. Neurotoxicol Teratol. 1992;14:259–64. [DOI] [PubMed] [Google Scholar]
- 44. Ribes D, Colomina MT, Vicens P, Domingo JL. Effects of oral aluminum exposure on behavior and neurogenesis in a transgenic mouse model of Alzheimer's disease. Exp Neurol. 2008;214:293–300. [DOI] [PubMed] [Google Scholar]
- 45. Sethi P, Jyoti A, Singh R, Hussain E, Sharma D. Aluminium‐induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. Neurotoxicology. 2008;29:1069–79. [DOI] [PubMed] [Google Scholar]
- 46. Yumoto S, Nagai H, Matsuzaki H, et al. Transplacental passage of 26Al from pregnant rats to fetuses and 26Al transfer through maternal milk to suckling rats. Nucl Instrum Methods Phys Res, Sect B. 2000;172:925–9. [Google Scholar]
- 47. Yumoto S, Nagai H, Matsuzaki H, et al. Aluminium incorporation into the brain of rat fetuses and sucklings. Brain Res Bull. 2001;55:229–34. [DOI] [PubMed] [Google Scholar]
- 48. Yokel RA, Hicks CL, Florence RL. Aluminum bioavailability from basic sodium aluminum phosphate, an approved food additive emulsifying agent, incorporated in cheese. Food Chem Toxicol. 2008;46:2261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Długaszek M, Fiejka MA, Graczyk A, Aleksandrowicz JC, Słowikowska M. Effects of various aluminium compounds given orally to mice on Al tissue distribution and tissue concentrations of essential elements. Pharmacol Toxicol. 2000;86:135–9. [DOI] [PubMed] [Google Scholar]
- 50. Şahin G, Varol I, Temizer A, Benli K, Demirdamar R, Duru S. Determination of aluminum levels in the kidney, liver, and brain of mice treated with aluminum hydroxide. Biol Trace Elem Res. 1994;41:129–35. [DOI] [PubMed] [Google Scholar]
- 51. Sánchez‐Iglesias S, Soto‐Otero R, Iglesias‐Gonzalez J, Barciela‐Alonso MC, Bermejo‐Barrera P, Méndez‐Álvarez E. Analysis of brain regional distribution of aluminium in rats via oral and intraperitoneal administration. J Trace Elem Med Biol. 2007;21:31–4. [DOI] [PubMed] [Google Scholar]
- 52. Nehru B, Anand P. Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J Trace Elem Med Biol. 2005;19:203–8. [DOI] [PubMed] [Google Scholar]
- 53. Campbell A, Becaria A, Lahiri DK, Sharman K, Bondy SC. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J Neurosci Res. 2004;75:565–72. [DOI] [PubMed] [Google Scholar]
- 54. Platt B, Fiddler G, Riedel G, Henderson Z. Aluminium toxicity in the rat brain: histochemical and immunocytochemical evidence. Brain Res Bull. 2001;55:257–67. [DOI] [PubMed] [Google Scholar]
- 55. Yokel RA, O'Callaghan JP. An aluminum‐induced increase in GFAP is attenuated by some chelators. Neurotoxicol Teratol. 1998;20:55–60. [DOI] [PubMed] [Google Scholar]
- 56. Tsunoda M, Sharma RP. Modulation of tumor necrosis factor α expression in mouse brain after exposure to aluminum in drinking water. Arch Toxicol. 1999;73:419–26. [DOI] [PubMed] [Google Scholar]
- 57. Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Müller N, Schwarz MJ. The immune‐mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. [DOI] [PubMed] [Google Scholar]
- 60. Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46:1724–43. [DOI] [PubMed] [Google Scholar]
- 61. Wang M, Chen JT, Ruan DY, Xu YZ. The influence of developmental period of aluminum exposure on synaptic plasticity in the adult rat dentate gyrus in vivo. Neuroscience. 2002;113:411–9. [DOI] [PubMed] [Google Scholar]
- 62. Kumar S. Aluminium‐induced changes in the rat brain serotonin system. Food Chem Toxicol. 2002;40:1875–80. [DOI] [PubMed] [Google Scholar]
- 63. El‐Rahman SSA. Neuropathology of aluminum toxicity in rats (glutamate and GABA impairment). Pharmacol Res. 2003;47:189–94. [DOI] [PubMed] [Google Scholar]
- 64. Nayak P, Chatterjee AK. Effects of aluminium exposure on brain glutamate and GABA systems: an experimental study in rats. Food Chem Toxicol. 2001;39:1285–9. [DOI] [PubMed] [Google Scholar]
- 65. Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant‐related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–23. [DOI] [PubMed] [Google Scholar]
- 66. McFadyen MP, Kusek G, Bolivar VJ, Flaherty L. Differences among eight inbred strains of mice in motor ability and motor learning on a rotorod. Genes Brain Behav. 2003;2:214–9. [DOI] [PubMed] [Google Scholar]
- 67. Mogil JS, Ritchie J, Sotocinal SG, et al. Screening for pain phenotypes: analysis of three congenic mouse strains on a battery of nine nociceptive assays. Pain. 2006;126:24–34. [DOI] [PubMed] [Google Scholar]
- 68. Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes Brain Behav. 2008;7:496–505. [DOI] [PubMed] [Google Scholar]
- 69. O'Leary TP, Gunn RK, Brown RE. What are we measuring when we test strain differences in anxiety in mice? Behav Genet. 2013;43:34–50. [DOI] [PubMed] [Google Scholar]
- 70. Võikar V, Kõks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–81. [DOI] [PubMed] [Google Scholar]
- 71. Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou‐Daifoti Z, Dalla C. Forced swim test: what about females? Neuropharmacology. 2015;99:408–21. [DOI] [PubMed] [Google Scholar]
- 72. Bangasser DA, Valentino RJ. Sex differences in stress‐related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey I: lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. [DOI] [PubMed] [Google Scholar]
- 74. Frick KM, Burlingame LA, Arters JA, Berger‐Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 1999;95:293–307. [DOI] [PubMed] [Google Scholar]
- 75. Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–91. [DOI] [PubMed] [Google Scholar]
- 76. Gresack JE, Frick KM. Male mice exhibit better spatial working and reference memory than females in a water‐escape radial arm maze task. Brain Res. 2003;982:98–107. [DOI] [PubMed] [Google Scholar]
- 77. LaBuda CJ, Mellgren RL, Hale RL. Sex differences in the acquisition of a radial maze task in the CD‐1 mouse. Physiol Behav. 2002;76:213–7. [DOI] [PubMed] [Google Scholar]
- 78. Shaw CA, Li Y, Tomljenovic L. Administration of aluminium to neonatal mice in vaccine‐relevant amounts is associated with adverse long term neurological outcomes. J Inorg Biochem. 2013;128:237–44. [DOI] [PubMed] [Google Scholar]
- 79. Shaw CA, Petrik MS. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J Inorg Biochem. 2009;103:1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


