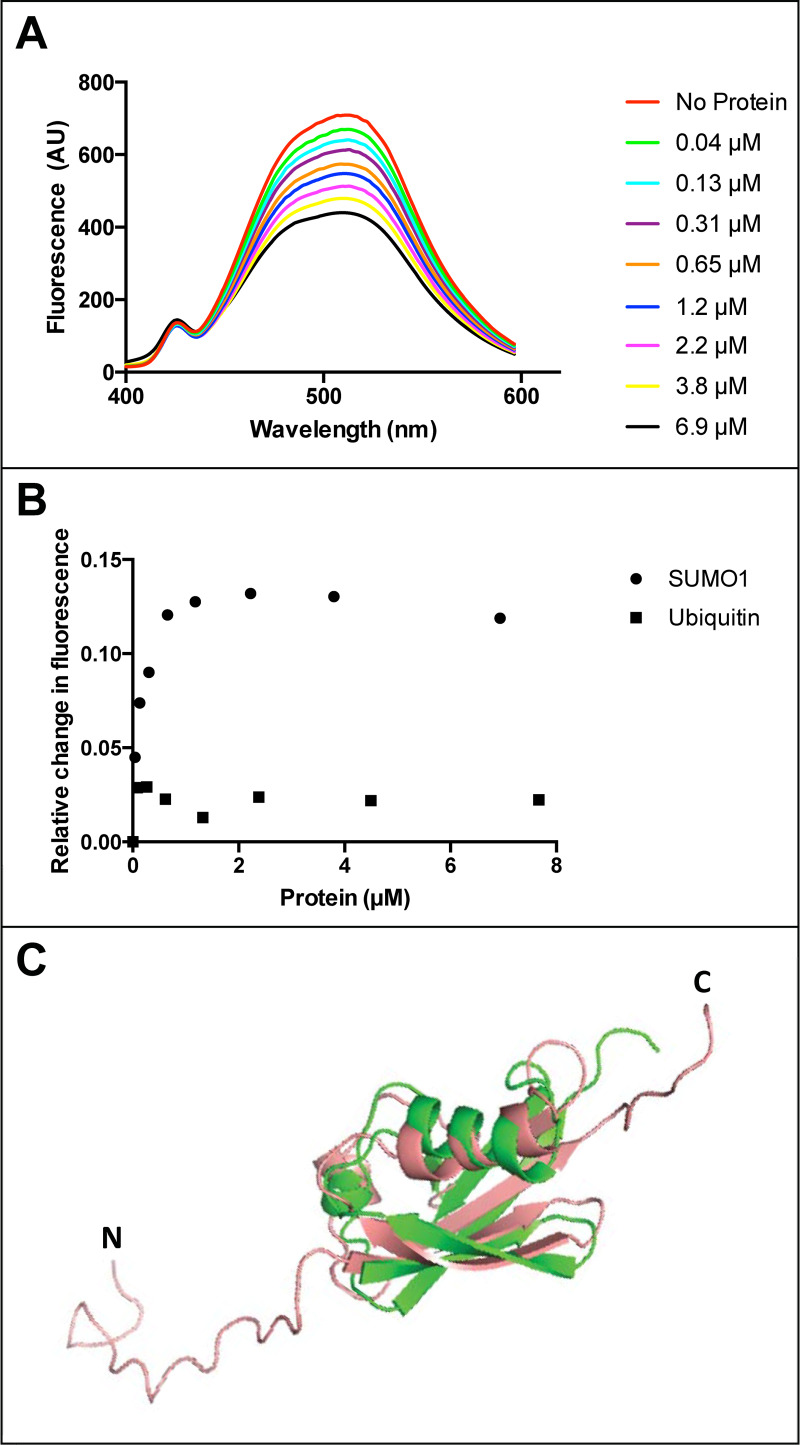
Fig 3. Fluorescence quenching of fisetin with human SUMO1.
(A) Fluorescence emission spectra of fisetin indicate quench in fluorescence upon addition of increasing concentrations of purified human SUMO1 protein. (B) Relative change in fluorescence of fisetin (i.e. relative quench of fisetin) after correction for the solvent buffer is plotted against concentration of human SUMO1 (circles) or bovine ubiquitin (squares). For SUMO1, the relative quench of fisetin fluorescence appears saturable. In contrast, ubiquitin addition results in a lower extent of fluorescence quenching. A representative graph is shown from two independent experiments in which a range of SUMO1 and ubiquitin concentrations were tested. (C) Pairwise alignment of SUMO1 (salmon, PDB 1A5R) and ubiquitin (green, PDB 1UBQ) using PyMOL2 illustrates structural 3D-fold conservation despite only ~18% amino acid identity. Amino (N) and carboxyl (C) termini of SUMO1 are indicated.