Abstract
Zebrafish have the ability to regenerate damaged cells and tissues by activating quiescent stem and progenitor cells or reprogramming differentiated cells into regeneration-competent precursors. Proliferation among the cells that will functionally restore injured tissues is a fundamental biological process underlying regeneration. Midkine-a is a cytokine growth factor, whose expression is strongly induced by injury in a variety of tissues across a range of vertebrate classes. Using a zebrafish Midkine-a loss of function mutant, we evaluated regeneration of caudal fin, extraocular muscle and retinal neurons to investigate the function of Midkine-a during epimorphic regeneration. In wildtype zebrafish, injury among these tissues induces robust proliferation and rapid regeneration. In Midkine-a mutants, the initial proliferation in each of these tissues is significantly diminished or absent. Regeneration of the caudal fin and extraocular muscle is delayed; regeneration of the retina is nearly completely absent. These data demonstrate that Midkine-a is universally required in the signaling pathways that convert tissue injury into the initial burst of cell proliferation. Further, these data highlight differences in the molecular mechanisms that regulate epimorphic regeneration in zebrafish.
Introduction
Epimorphic regeneration is the process of replacing ablated cells and tissues, which are then functionally integrated into the mature organ. The abiding scientific interest in epimorphic regeneration is sustained by the striking dichotomy in the regenerative abilities between vertebrates, such as amphibians and teleost fish, and mammals [1,2]. Further, identifying the molecular mechanisms that govern epimorphic regeneration holds the promise of informing therapeutic approaches for treating injuries in humans.
Zebrafish is an excellent model to study epimorphic regeneration. This teleost fish has the ability to regenerate multiple tissues, including fins, somatic muscle, heart muscle, and the central nervous system [3–5]. Following amputation, the caudal fin regenerates from intra-ray mesenchymal stem and progenitor cells and dedifferentiated osteoblasts [6–9]. This process is characterized by the formation of a proliferative blastema at the wound plane, which is capable of fully reconstructing the missing tissues [10]. The regenerative blastema can originate from resident, tissue-specific stem cells or extant mature cells that are reprogrammed into a dedifferentiated state [11,12]. Following ablation of muscle, myocytes dedifferentiate and enter the cell cycle to proliferate and regenerate functional tissue [7,13,14,15].
In contrast to fin and muscle, where injury reprograms extant cells into tissue-specific progenitors [16,17], regeneration in the central nervous system of zebrafish is sustained by radial glia, which also function as intrinsic neuronal stem cells [5,18–20]. In the retina, Müller glia are the intrinsic stem cells [21]. In response to cell death, Müller glia dedifferentiate, enter the cell cycle, and undergo a single asymmetric division to produce rapidly dividing, multipotent progenitors that continue to divide and differentiate into all types of retinal neurons [22,23]. Cell death also accelerates proliferation of rod precursors that are derived from Müller glia and that contribute genesis of rod photoreceptors [24–27].
Midkine is an evolutionarily conserved, heparin binding cytokine growth factor that in vertebrates has multiple functions during development, tissue repair, and disease [28–30]. During embryonic development in mammals, Midkine is highly expressed in proliferative cells, then rapidly downregulated at mid-gestation [31]. In adults, injuries in a variety of tissues strongly induce re-expression of Midkine, suggesting a universal function of Midkine during tissue injury, repair or regeneration [31–34]. During development in zebrafish, midkine-a, one of two paralogous midkine genes, is expressed in differentiating somites and the central nervous system [66]. In adults, midkine-a is induced during regeneration of the heart [35], fin [36], skeletal muscle [14] and retina [37,38]. Previously, we generated a Midkine-a-loss of function mutant, mdkami5001 [39]. Mdkami5001 larvae progress normally through early embryonic stages. Minor phenotypic changes are apparent at 48 hours post fertilization (hpf), when mutants display a slight reduction in body pigmentation, shortened body length, and smaller eyes, suggesting a slightly slower growth rate during larval stages. Adult mutants are viable and fertile and show complete phenotypic penetrance during regeneration (see below).
Following the selective ablation of photoreceptors in the mdkami5001 mutants, Müller glia enter the cell cycle, but fail to progress from G1 to S phases. As a consequence, cone photoreceptors do not regenerate [39]. The function of Midkine-a in zebrafish during the regeneration of somatic tissues and following other retinal injury paradigms has not been elucidated.
Using the Midkine-loss of function mutant [39], we compared the injury-induced proliferation and regeneration of three different tissues: caudal fin, extraocular muscle and retina. In the absence of Midkine-a, the initial proliferative response following injury to the caudal fin and extraocular muscle is significantly diminished. In contrast, following ablation of retinal neurons, proliferation is nearly absent, resulting in the failure of regeneration. These results demonstrate that Midkine-a governs the proliferative response in all forms of epimorphic regeneration and highlights differences in the cellular requirements for this injury-induced molecule.
Materials and methods
Animals
Fish were maintained at 280 C on a 14/10 hours light/ dark cycle, using standard husbandry procedures. AB wildtype (Danio rerio), mdkami5001, Tg(α-actin:mCherry) and mdkami5001;Tg(α-actin:mCherry) of either sex were used at 6 to 12 months of age. Within experiments, the ratio of males and females were closely matched. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Michigan and Wayne State University.
Anesthesia
Adult zebrafish were anesthetized using pharmaceutical grade/ FDA-approved MS-222/ Tricaine S (0.02%/ 20 mg/ 100ml, Western Chemical Inc., Ferndale, WA) dissolved in system water containing 0.02% sodium bicarbonate. Rapid chilling/ hypothermia and cervical transaction were used to sacrifice fish.
Lesion paradigms and labeling of proliferative cells
Fin amputation
Fish were anesthetized, and the distal portion of the caudal fins were amputated proximal to the first lepidotrichia branching point. Following amputation, fish were revived and returned to the housing system. All fin amputations were perpendicular to the anterior/posterior plane to avoid uneven fin outgrowth from the dorsal or ventral halves of the fin. Experimental fins were imaged prior to amputation, and at 4, 6, 12, 19, and 32 days post amputation (dpa). For the proliferation assay, fish were immersed in a 5 mM BrdU solution (Sigma Aldrich, St. Louis, MO) from 3 to 4 dpa as previously described [40].
Partial resection of extraocular muscle
Adult fish were anesthetized, and approximately 50% of one lateral rectus muscle was surgically excised [13]. To visualize extraocular muscle in situ, lesions were performed in Tg(α-actin:mCherry) and mdkami5001;Tg(α-actin:mCherry) lines. To label proliferating cells, fish were intraperitoneally injected with 20 μL of 10 mM EdU (Thermo Fisher Scientific, Waltham, MA) diluted in PBS at 20 hours following injury and sacrificed at 24 hours post injury.
Retinal lesions
Retinal neurons were ablated using either a mechanical stab [41] or an intraocular injection of the Na/K-ATPase inhibitor, ouabain (Sigma Aldrich). Briefly, mechanical injuries were performed by inserting a 30-gage needle into the dorsal aspect of the eyes through the sclera. Low doses of ouabain were used to selectively kill inner retinal neurons. For this, 0.5 μL of a 5 μM ouabain solution, diluted in PBS, was injected into the intravitreal space [22,42,43]. The contralateral, control eye received an injection of PBS. To assay for cell proliferation, fish were housed for 48 hrs in 5 mM BrdU between days 3 and 5 following the ouabain injection.
Tissue processing
Fins were harvested at 4 days post amputation (dpa) and were fixed in a 9:1 absolute ethanol (Fisher Scientific) and 37% formaldehyde solution (Sigma Aldrich). The tissues were infiltrated and frozen in tissue freezing medium (TFM, General Data Company, Cincinnati, OH; [44]). Radial cryosections were cut at 15 μm and mounted on glass slides.
Extraocular muscles were fixed in situ in buffered 4% paraformaldehyde (PFA, Fisher Scientific, Waltham, MA) and the cranium was decalcified using Morse’s solution [13]. 12 μm cryosections through the muscles, eyes, and skull were mounted on glass slides.
Eyes were fixed in either 9:1 ethanolic formaldehyde (100% ethanol: 37% formaldehyde) or buffered 4% PFA overnight at 4C0 [38,45,46]. All eyes were cryopreserved, embedded in freezing medium, and retinal sections were mounted on glass slides.
Immunohistochemistry and EdU detection
To visualize BrdU or PCNA, antigen retrieval was first performed using sodium citrate buffer (10 mM sodium citrate, 0.05% Tween-20, pH 6.0) as previously described [38,40,45]. Sections were then incubated in the primary antibody (Rat anti-BrdU [BU1/75(ICR1)], Abcam, ab6326, Cambridge, United Kingdom and Accurate Chemical and Scientific, OBT0030, Westbury, NY; Mouse anti-PCNA (clone PC 10), P8825, Sigma Aldrich; Mouse anti-HuC/D (16A11), A21271, Invitrogen, Carlsbad, CA) overnight at 4°C. After washing with PBST, sections were incubated in secondary antibodies (Alexa Fluor-conjugated 488, 594, and 647 goat anti-primary, Thermo Fisher Scientific) for 1 hour at room temperature. Nuclei were stained with either DAPI (Thermo Fisher Scientific) or TO-PRO-3 (Thermo Fisher Scientific). Fluorescence images were captured using a Leica TCS SP5 or SP8 confocal microscope (Leica Microsystems, Werzler, Germany). To visualize EdU-labelled cells, a Click-iT Assay kit (Thermo Fisher Scientific) was used.
Cell counts and outgrowth measurement
For fins, BrdU-labeled cells within the distal-most 400 microns of the blastemal compartment were counted. The counts in 3 different blastemas from each fish were averaged (n = 5 wildtype and 5 mutants). For lateral rectus muscles, EdU- and DAPI-labeled nuclei were counted and averaged in 4 to 6 non adjacent sections (n = 4 wildtype and 4 mutants). In stab lesioned retina, PCNA-positive cells in three non-adjacent sections were counted and averaged in each retina (n = 3 wildtype and 3 mutants). In ouabain injected retinas, BrdU-labeled cells were counted in the central dorsal retina in 3 non-adjacent sections per retina (n = 9 wildtype and 9 mutant) and the values were averaged. For outgrowth of the fin, each individual fin was imaged throughout regeneration at multiple time points (pre-amputation, 4, 6, 12, 19, and 32 dpa). Area of fin was calculated using ImageJ software (https://imagej.nih.gov/ij/). Percentage of the outgrowth was obtained from dividing the post-amputation area by the pre-amputation area and multiplied the result by 100.
qPCR
RNA was prepared from muscle tissue using TRIzol (Invitrogen), following the manufacturer’s protocol. Each sample was pooled from a total of 10 unlesioned (control) and 10 lesioned muscles. Three independent samples from control and lesioned muscles were then used to quantify gene expression. Following DNase treatment, RNA was quantified using a Nanodrop spectrophotometer (Thermo Fisher Scientific). RNA quality was assessed using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). RNA samples were reverse transcribed using an iScript reverse transcription kit (Bio-Rad Laboratories, Hercules, CA).
Gene expression was measured using CFX96 Real-Time PCR detection system (Bio-Rad Laboratories). Diluted cDNA, SsoFast EvaGreen supermix (Bio-Rad Laboratories), and specific primers were used in 20 μl. Reactions were performed in triplicate. The specificity of the PCR products was verified by analysis of melting curves and by electrophoresis. Gene expression was calculated by the ΔΔ C(t) method [47] using 18S rRNA [48] as the reference gene.
Statistical analysis
Statistical significance within data sets was analyzed using either the Student t-test or one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparisons test. All statistical tests were performed using the Prism 6.03 software for Mac OS X (GraphPad Software, Inc. La Jolla, CA). A p-value ≤ 0.05 was considered statistically significant.
Results
After an initial delay, Midkine-a mutants completely regenerate fins
Zebrafish regenerate caudal fins from the activation of intra-ray mesenchymal stem and progenitor cells and dedifferentiated osteoblasts [6–9]. RNAseq and gene expression analyses identified midkine-a as a gene that is strongly upregulated following fin amputation [36]. To determine the function of Midkine-a during fin regeneration, fins from wildtype and mdkami5001 were amputated and proliferation was evaluated in each using immunostaining for proliferating cell nuclear antigen (PCNA) and BrdU incorporation. In wildtype animals, the formation and outgrowth of the blastema was observed by 4 dpa (Fig 1A). Compared with wildtypes, the initial outgrowth of fins in the mdkami5001 was reduced in size (Fig 1A–1D), and the blastemal compartment contained significantly fewer PCNA- (Fig 1C–1F) and BrdU-positive cells (Fig 1G–1I). The reduced number of PCNA- and BrdU-labeled cells in the mutants may reflect delayed entry into the cell cycle or slower progression from G1 to S phase, as previously described for the retina ([39]; see discussion).
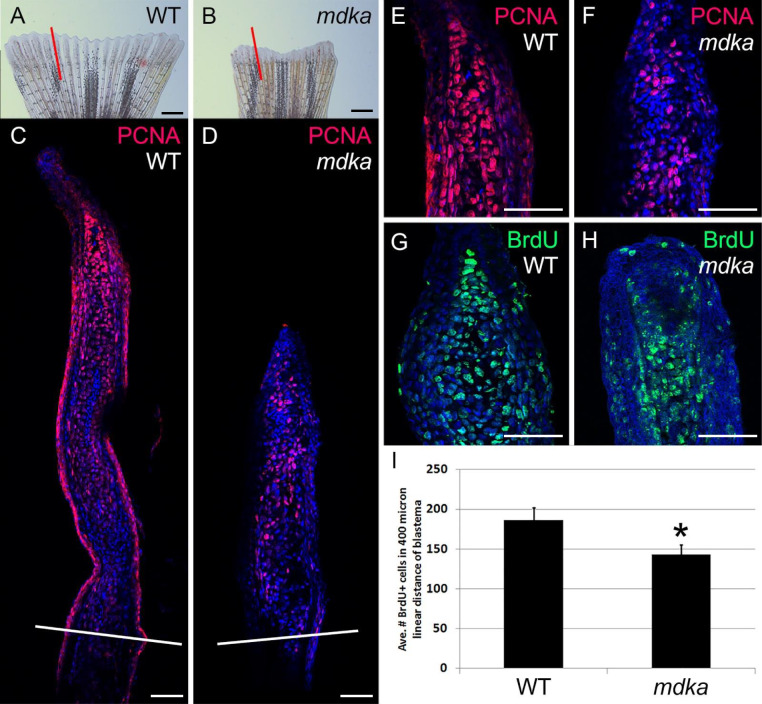
Fig 1. Initial proliferation is diminished in mdkami5001 following fin amputation.
(A-D) Stereo and confocal microscope images of regenerating fins in wildtype (A,C) and mdkami5001 (B,D), immunolabeled with PCNA (magenta). Red lines (A,B) indicate the plane of cross section shown in panels C and D. White lines across the fins show the levels of the amputation plane (C,D). Scale bars equal 400 μm in (A,B) and 50 μm in (C,D). (E-H) Confocal images of the blastemal compartments of fins from wildtype (E,G) and mdkami5001 (F,H), immunolabeled with PCNA (E,F) or BrdU (G,H) incorporated between 3–4 dpa (see Materials and Methods). Scale bars equal 50 μm in (E-H). (I) Graph illustrating the average number of BrdU-positive cells within a 400 mm linear distance. wildtype: 186.8 ± 15.1 cells, mdkami5001: 142.9 ± 12.3 cells, Student’s t-test, p = 0.0326, n = 5 wildtype and 5 mutants.
To determine if the diminished initial proliferation in the mdkami5001 led to persistently altered regeneration, the area of the regenerated tissue was measured over time. In wildtype animals, regeneration of the caudal fin was completed by 32 dpa (Fig 2A). Whereas outgrowth of the regenerated fin in mdkami5001 showed a significant reduction in area at 4 dpa, there was no statistically-significant difference in the area of the regenerated fins between wildtype and mdkami5001 at the subsequent the time points (Fig 2A–2C). Although the areas of regenerated fins were statistically indistinguishable, regenerated fins in mutants frequently displayed abnormal shapes and mis-patterned pigmentation (Fig 2A). These results indicate that Midkine-a is required initially to amplify proliferation among the population of cells that create the blastema. Recovery of the fin outgrowth suggests that in the absence of Midkine-a proliferation accelerates beyond 4 dpa, and this is sufficient to rescue the initial defect of proliferation. This also implies that once the blastemal is formed, Midkine-a is not required to complete regeneration. Further, we infer that the structural defects in regenerated fins in mdkami5001 are a consequence of the initially malformed blastema that is then propagated throughout the period of regeneration.
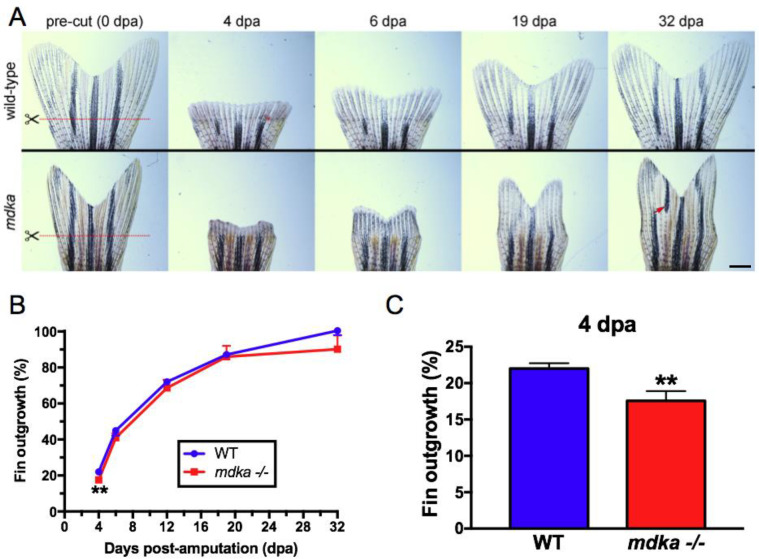
Fig 2. Initial fin regeneration is delayed but recovers in mdkami5001.
(A) Fins from wildtype (top) and mdkami5001 (bottom) at 0, 4, 6, 19, and 32 dpa. Red lines indicate the level of the amputation plane. In wildtype animals, the structure of the bony fin rays and pigmentation recover completely. Compared to age-matched group of wildtype, pre-amputated fin in the mdkami5001 is smaller in size (wildtype: 51.3 ± 7.1 μm2; mdkami5001: 34.3 ± 4.7 μm2, p<0.0001, Student’s t-test). In the mutants, regenerated fins display abnormal shape and mis-patterned melanophore pigmentation (red arrow). Scale bar equals 400 μm. (B) Graph illustrating the average percent outgrowth through 32 dpa, calculated from the ratio of the areas of pre- and post-amputation fins. Wildtype fins recover to 100% of pre-amputation levels by 32 dpa. The mutant animals show a significant reduction of fin outgrowth at 4 dpa. At the subsequent time points, there is no statistically significant difference in the fin outgrowth between wildtype and mdkami5001. (C) Graph illustrating the percent fin outgrowth at 4 dpa in wildtype and mdkami5001. Student’s t-test, p = 0.0105. n = 9 wildtype and 8 mutants.
In Midkine-a mutants, proliferation and regeneration of extraocular muscle is impaired
In zebrafish, regeneration of extraocular muscles utilizes the dedifferentiation and proliferation of extant myocytes, which then differentiate into muscle cells [7,13,14,48–50]. An RNAseq screen previously showed the upregulation of midkine-a during regeneration of extraocular muscle [14]. This result was validated, and it was determined that at 48 hours post lesion (hpl) there is a significant induction of midkine-a in the residual stump of muscle (Fig 3A). Compared with wildtype animals, at 24 hpl the proportion of the EdU-labeled cells in the mdkami5001 is significantly reduced (Fig 3B and 3C), consistent with the early requirement of Midkine-a during regeneration of the fin (see above). Measurements of the muscle growth revealed a significant initial delay in regeneration in the mdkami5001 (Fig 3D and 3E). These results indicate that, similar to regeneration of the caudal fin, Midkine-a is required for the initial proliferation in muscle induced by an injury.
Fig 3. Regeneration of the extraocular muscle is delayed in mdkami5001.
(A) qPCR for midkine-a in wildtype zebrafish at 48 hours post lesion. Student’s t-test, p*<0.05, n = 3 uninjured and 3 injured (10 muscles per sample). (B) Cell counts of EdU labeled cells in muscles at 24 hours post lesion from wildtype (n = 4) and mdkami5001(n = 4). Student’s t-test, p*<0.05. (C) Diagram of the craniectomized head of an adult zebrafish. Red lines identify the lateral rectus muscles. Excised muscle is represented as red dashed line. Asterisk identifies the pituitary body at the midline. Boxes represent areas illustrated in panels D and E. (D) EdU-labeled cells in muscles from wildtype (top) and mdkami5001 (bottom) at 24 hours post lesion. High magnification inset image illustrates an elongated myonucleus undergoing proliferation (also see [13]). Arrows indicate the growing tip of the regenerating muscle. Asterisk indicates the pituitary body at the midline. (E) Extraocular muscles are outlined by dashed lines in control animals and in mutants at 4 days post lesion. Asterisks identify the pituitary body. (F) Outgrowth of regenerating muscles at post-injury time points in wildtype and mdkami5001. One-way ANOVA followed by Newman-Keuls multiple comparisons test, p*<0.05, p**<0.01, n = 4 wildtype and 4 mutants.
Proliferation of Müller glia is diminished in Midkine-a mutants
A recent study demonstrated that, following selective ablation of photoreceptors, Midkine-a is required for Müller glia to progress through the cell cycle [39]. We first confirmed this previous finding using a stab wound lesion, which locally ablates all retinal neurons [51]. In wildtype animals, the proliferation of Müller glia and Müller glia-derived progenitors, which originate in the inner nuclear layer, increases at 48–72 hpl (Fig 4A and 4B). However, in the Midkine-a mutants at 48 and 72 hpl, the number of PCNA-positive cells was significantly less (Fig 4C, 4D, 4E, 4F and 4G).
Fig 4. Proliferation of Müller glia is compromised following a stab wound in mdkami5001.
(A-D) Immunocytochemistry for proliferative marker, PCNA, in wildtype (A,B) and mdkami5001 (C,D) at post-lesion time points. (E-G) Graphs illustrating the number of PCNA-positive cells in the outer nuclear layer (E; 48 hpl, wildtype: 20.9 ± 3.7 cells, mdkami5001: 2.6 ± 2.1 cells, p = 0.0016; 72 hpl, wildtype: 18.5 ± 5.8 cells, mdkami5001: 3.8 ± 1.1 cells, p = 0.0126), inner nuclear layer (F; 48 hpl, wildtype: 50.7 ± 11.9 cells, mdkami5001: 4.4 ± 5.3 cells, p = 0.0036; 72 hpl, wildtype: 43.7 ± 9.7 cells, mdkami5001: 8.9 ± 7.3 cells, p = 0.0077), and total retinal layer (G; 48 hpl, wildtype: 72.1 ± 12.9 cells, mdkami5001: 7.1 ± 6.3 cells, p = 0.0014; 72 hpl, wildtype: 56.4 ± 19.1 cells, mdkami5001: 12.8 ± 8.0 cells, p = 0.0212). Scale bar equals 60 μm. Student’s t-test, p*<0.05, n = 3 wildtype and 3 mutants. ONL: outer nuclear layer; INL: inner nuclear layer.
We also used intraocular injection of low-dose ouabain, to selectively ablate inner retinal neurons [42]. These studies were performed to evaluate this lesion paradigm in the Midkine-a mutants and to determine if rod progenitors, which are spared by ouabain injections [52] and are proposed to be lineage-restricted [39], are capable of regenerating inner retinal neurons. The cell-type specific marker, HuC/D [22,42,53] and PCNA labeling were used to evaluate the ouabain lesions and initial proliferative response, respectively. At 72 hours post injection (hpi), compared with controls, ouabain dramatically reduced the number of HuC/D-positive cells, in both wildtype and mdkami5001 retinas, demonstrating that low-dose ouabain injections successfully ablate inner retinal neurons (Fig 5A–5C). Consistent with previous reports [43], photoreceptors in the outer retina are largely spared as evident by the normal lamination and cellular density within the outer nuclear layer (Fig 5A and 5B). In wildtype retinas at 72 hpi, elongated, PCNA-positive nuclei of Müller glia and Müller glia-derived progenitors are observed in the inner nuclear layer (Fig 5D). In contrast, at 72 hpi in the mdkami5001 retinas, very few PCNA positive cells are present in the inner retina (Fig 5E and 5F). The PCNA-positive cells present in the mutant retinas are likely either endothelial cells or activated microglia, which are also PCNA-positive in wildtype retinas [22,54]. These results indicate that in the mdkami5001, Müller glia fail to proliferate following ablation of inner retinal neurons, and that the extent of cell death or types of retinal neurons ablated does not alter the requirement of Midkine-a for Müller glia to progress through the cell cycle.
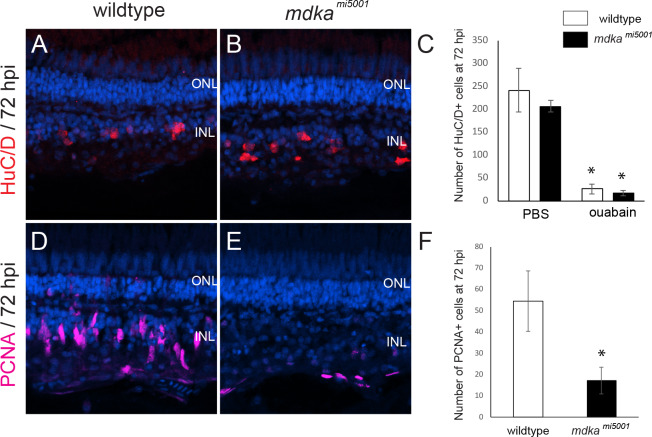
Fig 5. Absence of proliferation in mdkami5001 following the death of inner retinal neurons.
(A, B) Immunocytochemistry for the cell marker, HuC/D, in wildtype (A) and mdkami5001 (B) at 72 hpi. (C) The number of HuC/D-positive cells following the injection of PBS (wildtype: 241.3 ± 28.2 cells, mdkami50011: 206.0 ± 12.4 cells) and ouabain (wildtype: 25.8 ± 10.5 cells, mdkami5001: 17.2 ± 7.2 cells, p = 0.137) retinas. (D, E) Immunocytochemistry for proliferative marker, PCNA in wildtype (D) and mdkami5001 (E) at 72 hpi. (F) The number of PCNA-positive cells per 500 μm in wildtype (54.4 ± 14.2 cells) and mdkami5001 (17.1 ± 6.4 cells. p = 0.00246) at 72 hpi. Student’s t-test, p*<0.05, n = 9 wildtype and 9 mutants. ONL: outer nuclear layer; INL: inner nuclear layer.
In addition, animals were exposed to BrdU between 3 and 5 days post injection (dpi) and regenerated neurons were counted at 28 dpi. In wildtype retinas, BrdU-labeled neurons were scattered across all retinal layers, indicating regeneration of inner retinal neurons and a few photoreceptor cells (Fig 6A). BrdU-labeled nuclei of rod photoreceptors represent the ongoing proliferation of rod precursors following ouabain-induced damage. In contrast, there were significantly less BrdU-labeled cells in the mdkami5001 retinas (Fig 6B and 6C). Further, as a consequence of the diminished initial proliferation, at 28 dpi in the mdkami5001 there was a complete absence of the inner retinal layers (Fig 6D–6F). This striking result also demonstrates that rod progenitors are incapable of regenerating inner retinal neurons (see Nagashima et al., 2020), demonstrating that the fate of rod progenitors is restricted, and these cells are capable only of generating rod photoreceptors.
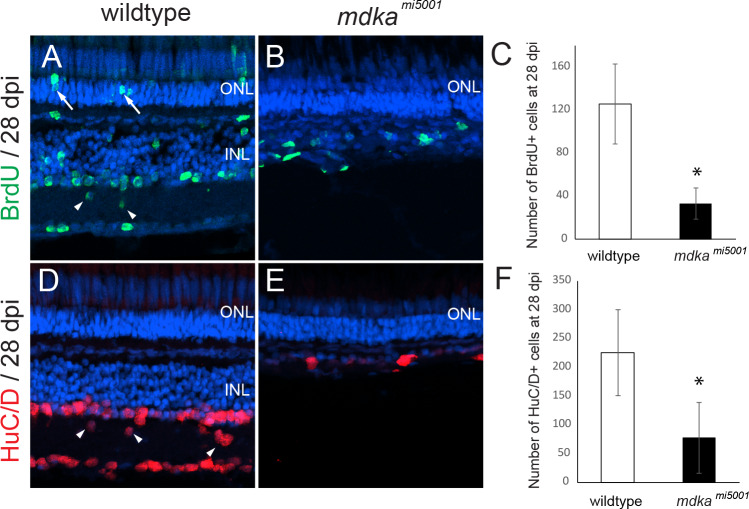
Fig 6. Absence of retinal regeneration in mdkami5001 at 28 dpl following the death of inner retinal neurons.
(A, B) BrdU-labeled regenerated retinal neurons in wildtype (A) and mdkami5001 (B) retinas at 28 dpi. Arrows indicated BrdU-labeled rod photoreceptors. (C) The number of BrdU-positive cells wildtype (125.2 ± 37.1 cells) and mdkami5001 (33.2 ± 14.4 cells, p = 0.000341) retinas at 28 dpi. (D,E) Regenerated inner neurons labeled with HuC/D in wildtype (D) and mdkami5001 (E) at 28 dpi. Arrowheads indicate regenerated inner neurons are displaced in the inner plexiform layer. (F) The number of HuC/D cells in wildtype (224.8 ± 74.6 cells) and mdkami5001 (76.8 ± 61.4 cells, p = 0.00021) at 28 dpi. Student’s t-test, p*<0.05, n = 9 wildtype and 9 mutants. ONL: outer nuclear layer; INL: inner nuclear layer.
Discussion
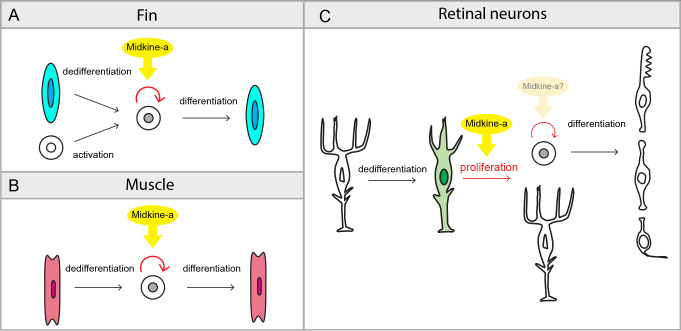
During epimorphic regeneration, mature, differentiated cells reprogram into stem and progenitor states [55,56]. Proliferation is a fundamental mechanism that serves to amplify these stem and progenitor cells in order to replace lost or damaged tissues. Our results demonstrate that Midkine-a, a soluble cytokine growth factor induced by tissue damage, governs elements of this proliferation during epimorphic regeneration (Fig 7).
Fig 7. Summary diagram: The function of Midkine-a during epimorphic regeneration.
(A,B) Following fin amputation, Midkine-a regulates proliferation of precursors produced from dedifferentiation of osteoblasts and activation of mesenchymal stem and progenitors (A). Following extraocular muscle excision, Midkine-a regulates proliferation of precursor produced from dedifferentiation of myocyte (B). During fin and muscle regeneration, loss of Midkine-a results in diminished initial burst of proliferation. (C) During regeneration of retinal neurons, Midkine-a governs proliferation of dedifferentiated Müller glia. In the absence of Midkine-a, retinal neurons fail to regenerate.
Regeneration is a multi-step process involving dedifferentiation, proliferation, and differentiation. Numerous factors and signaling pathways regulate different components of this biological process [57,58]. During the regeneration of both fin and extraocular muscle, Fgf signaling governs the formation of the blastema and the proliferation of dedifferentiated cells [50,59,60]. Similarly, during regeneration of the fin Igf signaling is required for the proliferation of blastema cells. In contrast, during the regeneration of muscle Igf is involved late, during the differentiation of newly generated myoblasts [49,61]. Our results demonstrate that the cytokine growth factor, Midkine-a, is also involved in tissue regeneration, possibly as a generic wound signal to regulate initial burst of proliferation during the regeneration of both fin and muscle (Fig 7A and 7B and [62]). Although the cell types in fin and muscle that express Midkine-a are not yet established, we infer that in these tissues Midkine-a functions as a paracrine and/or autocrine regulator of proliferation that acts on activated, dedifferentiated cells that give rise to the regeneration blastema.
There are at least two mechanisms that can explain the function of Midkine-a during the regeneration of fin, muscle, and retinal neurons. First, Midkine-a may be required for a subset of reprogrammed cells to enter into, or progress through the cell cycle. Cellular reprogramming occurs in all the tissues examined, and entering and/or progressing through the cell cycle is a universal requirement among reprogrammed cells. During regeneration of fin, proliferative cells in the blastemal are derived from multiple sources of reprogrammed cells, whereas during muscle regeneration myocytes are solely responsible to form the blastema. The initial delay of the burst of proliferation may reflect incomplete contribution among cells capable of forming the blastema. This scenario is consistent with that following light lesions, very few Müller glia are able to progress through the cell cycle [39]. Second, Midkine-a may govern cell cycle kinetics among progenitors derived from reprogrammed and/or stem-like cells. Midkine-a may be required for the amplification of this population of cells, perhaps serving to match the initial size of the blastema to the spatial extent of the injury. Utilizing murine embryonic stem cells [63], human cancer-derived cell lines [64], and the embryonic retina in zebrafish [65], it has been established that Midkine can govern cell cycle kinetics. Additional research will be required to determine which of these alternative mechanisms is correct.
In the intact teleost retina, rod precursors give rise to rod photoreceptors [24–26]. Our data are consistent with a previous report that in the Midkine-a mutants following selective ablation of photoreceptors, the regeneration of cone photoreceptors is permanently compromised, whereas rod photoreceptors are replenished (38). The failure to regenerate inner retinal neurons in the Midkine-a mutants indicates that the fate of rod precursors is restricted to the rod photoreceptor lineage. Given that rod precursors originate from Müller glia [18], this result suggests that following their migration to the outer retina a lineage restriction is imposed on these cells by the local environment within the outer nuclear layer. Our data also demonstrate that Midkine-a-mediated proliferation of Müller glia and generation of Müller glia-derived progenitors are required for regeneration of retinal neurons. This conclusion leads to the question: Does Midkine-a regulate proliferation of Müller glia-derived progenitors (Fig 7C)? The current manuscript does not investigate the question, however, we would predict two possible scenarios. First, in the mutants, proliferation of progenitors is also blocked. This would indicate conserved molecular machinery to regulate cell cycle among the cells that proliferate and Midkine-a plays a major role during the proliferation. Second, in the absence of Midkine-a, proliferation of progenitors is largely intact. This would imply that a different set of factors are required for proliferation of dedifferentiated cells versus progenitors. If the cells are already in the active cell cycle, Midkine-a may play a less role. We favor the second possibility, supporting previous observation that in the absence of Midkine-a, mutant animals progress slowly but normally through development and post-embryonic growth [39]. Additional studies will be needed to further understand cell type specific requirements of Midkine-a during proliferation.
Taken together, our data demonstrate that in zebrafish Midkine-a universally regulates the initial proliferation of reprogrammed cells and/or their progenitors (see also 38), which are activated following tissue damage. Further, our results highlight differences in the molecular mechanisms by which Midkine-a regulates epimorphic regeneration. The loss of Midkine-a diminishes regeneration of fin and extraocular muscle, whereas the absence of Midkine-a blocks regeneration of retinal neurons. These differences may reflect different properties of reprogrammed cells in different tissues. During regeneration of fin and extraocular muscle, osteoblasts and myocytes, respectively, reprogram into ‘late-stage’ progenitors that undergo multiple symmetric divisions [58]. In contrast, during regeneration of retinal neurons, Müller glia transiently reprogram into a retinal stem cell and undergo a single, asymmetric and self-renewing division [22]. Another possibility is that Midkine-b, which is structurally similar to Midkine-a [66], and/ or Pleiotrophin, a member of Midkine family of cytokine/growth factors [30], compensate the lack of Midkine-a in the fin and muscle, but not in the retina. Finally, it also remains to be determined if variability in receptors or intracellular signaling pathways may be responsible for the differences in the requirement of Midkine-a during the regeneration of different tissues.
Acknowledgments
The authors thank Dilip Pawar and Xixia Luo for zebrafish maintenance and technical assistance.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from the National Institutes of Health (NEI); R01EY07060 (to PFH) and R01EY026551 (to RT). Histology and imaging core resources were supported by vision core grants (P30EY07003 (to PFH) and P30EY04068 (to RT)) and an unrestricted grant from Research to Prevent Blindness to each department. Fish lines and reagents provided by ZIRC were supported by NIH-NCRR Grant P40 RR01. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310: 1919–1923. 10.1126/science.1115200 [DOI] [PubMed] [Google Scholar]
- 2.Muneoka K, Allan CH, Yang X, Lee J, Han M. Mammalian regeneration and regenerative medicine. Birth Defects Res C Embryo Today. 2008;84: 265–280. 10.1002/bdrc.20137 [DOI] [PubMed] [Google Scholar]
- 3.Gemberling M, Bailey TJ, Hyde DR, Poss KD. The zebrafish as a model for complex tissue regeneration. Trends Genet. 2013;29: 611–620. 10.1016/j.tig.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaźwińska A, Sallin P. Regeneration versus scarring in vertebrate appendages and heart. J Pathol. 2016;238: 233–246. 10.1002/path.4644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2012;72: 429–461. 10.1002/dneu.20918 [DOI] [PubMed] [Google Scholar]
- 6.Nakatani Y, Nishidate M, Fujita M, Kawakami A, Kudo A. Migration of mesenchymal cell fated to blastema is necessary for fish fin regeneration. Dev Growth Differ. 2008;50: 71–83. 10.1111/j.1440-169X.2007.00977.x [DOI] [PubMed] [Google Scholar]
- 7.Sousa S, Afonso N, Bensimon-Brito A, Fonseca M, Simões M, Leon J, et al. Differentiated skeletal cells contribute to blastema formation during zebrafish fin regeneration. Development. 2011;138: 3897–3905. 10.1242/dev.064717 [DOI] [PubMed] [Google Scholar]
- 8.Knopf F, Hammond C, Chekuru A, Kurth T, Hans S, Weber CW, et al. Bone Regenerates via Dedifferentiation of Osteoblasts in the Zebrafish Fin. Developmental Cell. 2011. pp. 713–724. 10.1016/j.devcel.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 9.Pfefferli C, Jaźwińska A. The art of fin regeneration in zebrafish. Regeneration (Oxf). 2015;2: 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan TH. Regeneration. 1901. 10.5962/bhl.title.87895 [DOI] [Google Scholar]
- 11.Sánchez Alvarado A, Tsonis PA. Bridging the regeneration gap: genetic insights from diverse animal models. Nat Rev Genet. 2006;7: 873–884. 10.1038/nrg1923 [DOI] [PubMed] [Google Scholar]
- 12.Antos CL, Tanaka EM. Vertebrates that regenerate as models for guiding stem cels. Adv Exp Med Biol. 2010;695: 184–214. 10.1007/978-1-4419-7037-4_13 [DOI] [PubMed] [Google Scholar]
- 13.Saera-Vila A, Kasprick DS, Junttila TL, Grzegorski SJ, Louie KW, Chiari EF, et al. Myocyte Dedifferentiation Drives Extraocular Muscle Regeneration in Adult Zebrafish. Invest Ophthalmol Vis Sci. 2015;56: 4977–4993. 10.1167/iovs.14-16103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louie KW, Saera-Vila A, Kish PE, Colacino JA, Kahana A. Temporally distinct transcriptional regulation of myocyte dedifferentiation and Myofiber growth during muscle regeneration. BMC Genomics. 2017;18: 854 10.1186/s12864-017-4236-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Geoffrey Burns C, et al. A Dynamic Epicardial Injury Response Supports Progenitor Cell Activity during Zebrafish Heart Regeneration. Cell. 2006. pp. 607–619. 10.1016/j.cell.2006.08.052 [DOI] [PubMed] [Google Scholar]
- 16.Sallin P, de Preux Charles A-S, Duruz V, Pfefferli C, Jaźwińska A. A dual epimorphic and compensatory mode of heart regeneration in zebrafish. Developmental Biology. 2015. pp. 27–40. 10.1016/j.ydbio.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 17.Wu C-C, Kruse F, Vasudevarao MD, Junker JP, Zebrowski DC, Fischer K, et al. Spatially Resolved Genome-wide Transcriptional Profiling Identifies BMP Signaling as Essential Regulator of Zebrafish Cardiomyocyte Regeneration. Developmental Cell. 2016. pp. 36–49. 10.1016/j.devcel.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 18.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci. 2007;27: 7028–7040. 10.1523/JNEUROSCI.1624-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26: 6303–6313. 10.1523/JNEUROSCI.0332-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008;68: 392–408. 10.1002/dneu.20596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25: 397–424. 10.1016/j.preteyeres.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 22.Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140: 4510–4521. 10.1242/dev.090738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenkowski JR, Raymond PA. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res. 2014;40: 94–123. 10.1016/j.preteyeres.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond PA, Rivlin PK. Germinal cells in the goldfish retina that produce rod photoreceptors. Dev Biol. 1987;122: 120–138. 10.1016/0012-1606(87)90338-1 [DOI] [PubMed] [Google Scholar]
- 25.Stenkamp DL. The rod photoreceptor lineage of teleost fish. Prog Retin Eye Res. 2011;30: 395–404. 10.1016/j.preteyeres.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232: 62–76. 10.1006/dbio.2001.0163 [DOI] [PubMed] [Google Scholar]
- 27.Morris AC, Scholz TL, Brockerhoff SE, Fadool JM. Genetic dissection reveals two separate pathways for rod and cone regeneration in the teleost retina. Dev Neurobiol. 2008;68: 605–619. 10.1002/dneu.20610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto K, Kadomatsu K. Midkine in the pathology of cancer, neural disease, and inflammation. Pathol Int. 2012;62: 445–455. 10.1111/j.1440-1827.2012.02815.x [DOI] [PubMed] [Google Scholar]
- 29.Winkler C, Yao S. The midkine family of growth factors: diverse roles in nervous system formation and maintenance. British Journal of Pharmacology. 2014. pp. 905–912. 10.1111/bph.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorrelle N, Dominguez ATA, Brekken RA. From top to bottom: midkine and pleiotrophin as emerging players in immune regulation. J Leukoc Biol. 2017;102: 277–286. 10.1189/jlb.3MR1116-475R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kadomatsu K, Huang RP, Suganuma T, Murata F, Muramatsu T. A retinoic acid responsive gene MK found in the teratocarcinoma system is expressed in spatially and temporally controlled manner during mouse embryogenesis. J Cell Biol. 1990;110: 607–616. 10.1083/jcb.110.3.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida Y, Sakakima H, Matsuda F, Ikutomo M. Midkine in repair of the injured nervous system. Br J Pharmacol. 2014;171: 924–930. 10.1111/bph.12497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato W, Yuzawa Y, Kadomatsu K, Tayasu T, Muramatsu H, Muramatsu T, et al. Midkine expression in the course of nephrogenesis and its role in ischaemic reperfusion injury. Nephrol Dial Transplant. 2002;17 Suppl 9: 52–54. [DOI] [PubMed] [Google Scholar]
- 34.Sakakima H, Yoshida Y, Muramatsu T, Yone K, Goto M, Ijiri K, et al. Traumatic injury-induced midkine expression in the adult rat spinal cord during the early stage. J Neurotrauma. 2004;21: 471–477. 10.1089/089771504323004610 [DOI] [PubMed] [Google Scholar]
- 35.Lien C-L, Schebesta M, Makino S, Weber GJ, Keating MT. Gene Expression Analysis of Zebrafish Heart Regeneration. PLoS Biology. 2006. p. e260 10.1371/journal.pbio.0040260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaźwińska A, Badakov R, Keating MT. Activin-betaA signaling is required for zebrafish fin regeneration. Curr Biol. 2007;17: 1390–1395. 10.1016/j.cub.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 37.Calinescu A-A, Vihtelic TS, Hyde DR, Hitchcock PF. Cellular expression of midkine-a and midkine-b during retinal development and photoreceptor regeneration in zebrafish. J Comp Neurol. 2009;514: 1–10. 10.1002/cne.21999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gramage E, D’Cruz T, Taylor S, Thummel R, Hitchcock PF. Midkine-a protein localization in the developing and adult retina of the zebrafish and its function during photoreceptor regeneration. PLoS One. 2015;10: e0121789 10.1371/journal.pone.0121789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagashima M, D’Cruz TS, Danku AE, Hesse D, Sifuentes C, Raymond PA, et al. Midkine-a Is Required for Cell Cycle Progression of Müller Glia during Neuronal Regeneration in the Vertebrate Retina. J Neurosci. 2020;40: 1232–1247. 10.1523/JNEUROSCI.1675-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ranski AH, Kramer AC, Morgan GW, Perez JL, Thummel R. Characterization of retinal regeneration in adult zebrafish following multiple rounds of phototoxic lesion. PeerJ. 2018;6: e5646 10.7717/peerj.5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Senut M-C, Gulati-Leekha A, Goldman D. An Element in the 1-Tubulin Promoter Is Necessary for Retinal Expression during Optic Nerve Regeneration But Not after Eye Injury in the Adult Zebrafish. Journal of Neuroscience. 2004. pp. 7663–7673. 10.1523/JNEUROSCI.2281-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27: 1712–1724. 10.1523/JNEUROSCI.5317-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas JL, Morgan GW, Dolinski KM, Thummel R. Characterization of the pleiotropic roles of Sonic Hedgehog during retinal regeneration in adult zebrafish. Exp Eye Res. 2018;166: 106–115. 10.1016/j.exer.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thummel R, Bai S, Sarras MP Jr, Song P, McDermott J, Brewer J, et al. Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev Dyn. 2006;235: 336–346. 10.1002/dvdy.20630 [DOI] [PubMed] [Google Scholar]
- 45.Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res. 2008;87: 433–444. 10.1016/j.exer.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranski AH, Kramer AC, Morgan GW, Perez JL, Thummel R. Characterization of retinal regeneration in adult zebrafish following multiple rounds of phototoxic lesion. PeerJ. 2018;6: e5646 10.7717/peerj.5646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001. pp. 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 48.Saera-Vila A, Kish PE, Louie KW, Grzegorski SJ, Klionsky DJ, Kahana A. Autophagy regulates cytoplasmic remodeling during cell reprogramming in a zebrafish model of muscle regeneration. Autophagy. 2016. pp. 1864–1875. 10.1080/15548627.2016.1207015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saera-Vila A, Louie KW, Sha C, Kelly RM, Kish PE, Kahana A. Extraocular muscle regeneration in zebrafish requires late signals from Insulin-like growth factors. PLoS One. 2018;13: e0192214 10.1371/journal.pone.0192214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saera-Vila A, Kish PE, Kahana A. Fgf regulates dedifferentiation during skeletal muscle regeneration in adult zebrafish. Cell Signal. 2016;28: 1196–1204. 10.1016/j.cellsig.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Senut M-C, -C. Senut M. An Element in the 1-Tubulin Promoter Is Necessary for Retinal Expression during Optic Nerve Regeneration But Not after Eye Injury in the Adult Zebrafish. Journal of Neuroscience. 2004. pp. 7663–7673. 10.1523/JNEUROSCI.2281-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raymond PA, Reifler MJ, Rivlin PK. Regeneration of goldfish retina: rod precursors are a likely source of regenerated cells. J Neurobiol. 1988;19: 431–463. 10.1002/neu.480190504 [DOI] [PubMed] [Google Scholar]
- 53.Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25: 143–155. 10.1002/neu.480250206 [DOI] [PubMed] [Google Scholar]
- 54.Mitchell DM, Lovel AG, Stenkamp DL. Dynamic changes in microglial and macrophage characteristics during degeneration and regeneration of the zebrafish retina. J Neuroinflammation. 2018;15: 163 10.1186/s12974-018-1185-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brockes JP, Kumar A. Comparative Aspects of Animal Regeneration. Annual Review of Cell and Developmental Biology. 2008. pp. 525–549. 10.1146/annurev.cellbio.24.110707.175336 [DOI] [PubMed] [Google Scholar]
- 56.Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3: 566–574. 10.1038/nrm881 [DOI] [PubMed] [Google Scholar]
- 57.Wehner D, Weidinger G. Signaling networks organizing regenerative growth of the zebrafish fin. Trends Genet. 2015;31: 336–343. 10.1016/j.tig.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 58.Sehring IM, Jahn C, Weidinger G. Zebrafish fin and heart: what’s special about regeneration? Curr Opin Genet Dev. 2016;40: 48–56. 10.1016/j.gde.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 59.Whitehead GG, Makino S, Lien C-L, Keating MT. fgf20 is essential for initiating zebrafish fin regeneration. Science. 2005;310: 1957–1960. 10.1126/science.1117637 [DOI] [PubMed] [Google Scholar]
- 60.Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, et al. Roles for Fgf signaling during zebrafish fin regeneration. Dev Biol. 2000;222: 347–358. 10.1006/dbio.2000.9722 [DOI] [PubMed] [Google Scholar]
- 61.Chablais F, Jazwinska A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development. 2010;137: 871–879. 10.1242/dev.043885 [DOI] [PubMed] [Google Scholar]
- 62.Owlarn S, Klenner F, Schmidt D, Rabert F, Tomasso A, Reuter H, et al. Generic wound signals initiate regeneration in missing-tissue contexts. Nat Commun. 2017;8: 2282 10.1038/s41467-017-02338-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao X, Tan Z, Gu B, Wu R-R, Liu Y-K, Dai L-C, et al. Promotion of self-renewal of embryonic stem cells by midkine. Acta Pharmacol Sin. 2010;31: 629–637. 10.1038/aps.2010.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mirkin BL, Clark S, Zheng X, Chu F, White BD, Greene M, et al. Identification of midkine as a mediator for intercellular transfer of drug resistance. Oncogene. 2005;24: 4965–4974. 10.1038/sj.onc.1208671 [DOI] [PubMed] [Google Scholar]
- 65.Luo J, Uribe RA, Hayton S, Calinescu A-A, Gross JM, Hitchcock PF. Midkine-A functions upstream of Id2a to regulate cell cycle kinetics in the developing vertebrate retina. Neural Dev. 2012;7: 33 10.1186/1749-8104-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkler C. Functional Divergence of Two Zebrafish Midkine Growth Factors Following Fish-Specific Gene Duplication. Genome Research. 2003. pp. 1067–1081. 10.1101/gr.1097503 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.