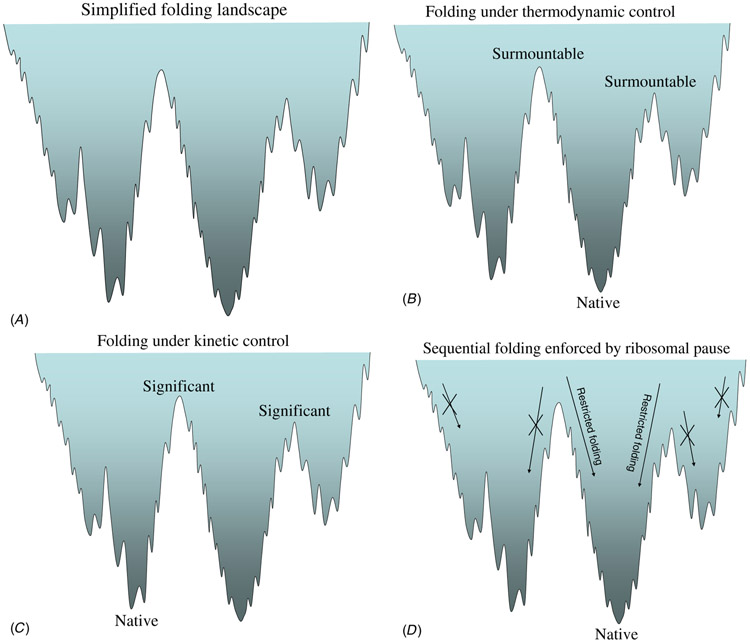
Figure 1.
Simplified free energy landscape to illustrate the folding complexity. Here, the folding landscape is depicted as a funnel shape containing three subdivided funnels with each distinguished by distinct folding pathways (figure 1(A)). If the free energy barriers between sub-funnels are surmountable and the protein native conformation is the corresponding global minimum, there is no misfolding but just various folding rates. Hence, this is the case referred to as protein folding under thermodynamic control (figure 1(B)). On the other hand, if the barriers are significant, misfoldings do happen when a folding path leads into a misfolded funnel. However, if the trapped conformation reflects the native protein, then this kind of protein folding is under kinetic control (figure 1(C)). A sequential folder aided by ribosomal pause to increase the chances of achieving a correctly folded protein is simply to maintain its folding pathways within the correct funnel (figure 1(D)).