Fig. 5. Evaporating droplets of collagen pattern mammalian cell alignment.
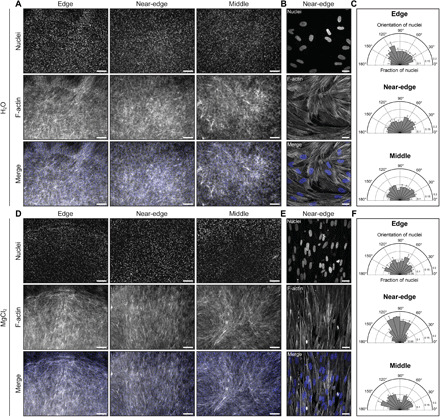
Representative (A) low and (B) high magnification fluorescence images of human skeletal muscle cells in each region of interest of a self-assembled droplet of collagen after 3 days in differentiation medium. The concentration of collagen was 2 mg/ml. RH was controlled using water (RH ~ 100%). (C) Orientation of nuclei in the edge (n = 2511), near-edge (n = 2148), and middle (n = 1767) regions of the droplet, where n represents the number of nuclei analyzed. Representative (D) low and (E) high magnification fluorescence images of human skeletal muscle cells in each region of interest of a self-assembled droplet of collagen after 3 days in differentiation medium. The concentration of collagen was ~2.3 mg/ml. RH was controlled using a saturated solution of MgCl2 (RH ~ 31%). (F) Orientation of nuclei in the edge (n = 2928), near-edge (n = 2381), and middle (n = 2638) regions of the droplet, where n represents the number of nuclei analyzed. Panels (B) and (E) represent the maximum-intensity z projection of a 7.8-μm-thick stack and a 10-μm-thick stack, respectively. Scale bars, 250 μm (A and D) and 25 μm (B and E). Cells are stained with Hoechst 33342 to label nuclei (blue) and phalloidin to label F-actin (gray). Collagen solutions were gelled on UVO-treated glass.
