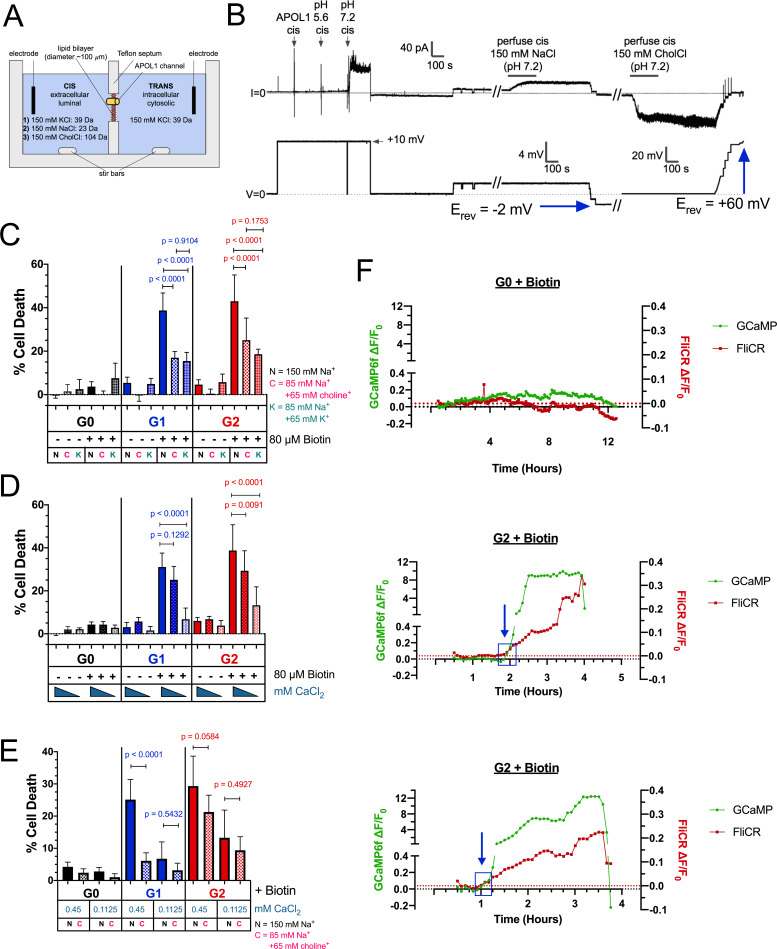
Figure 6. RRV cytotoxicity is driven by the influx of both Na+ and Ca2+.
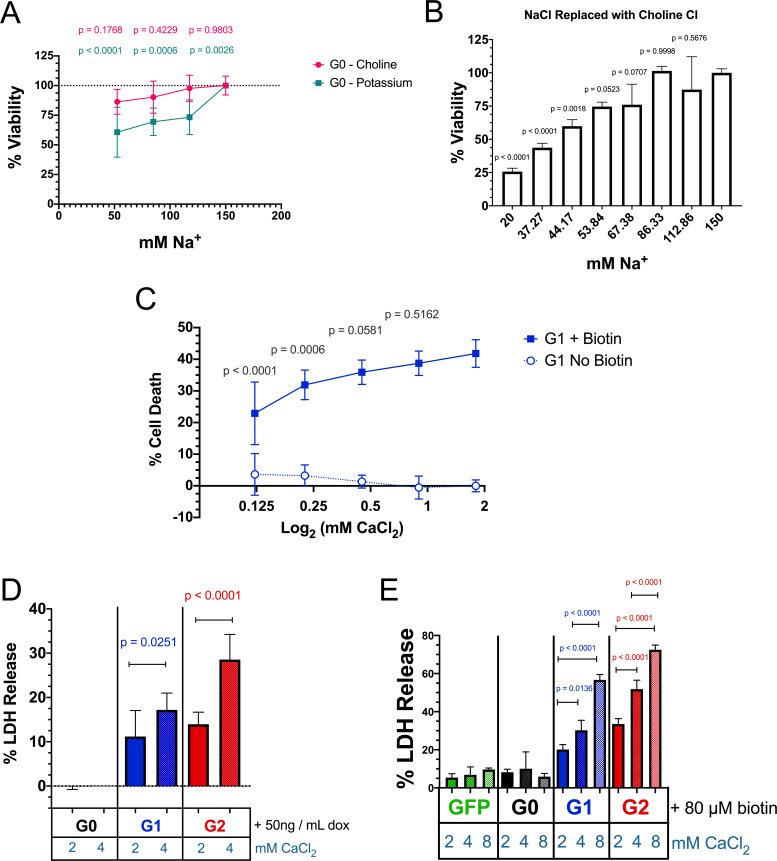
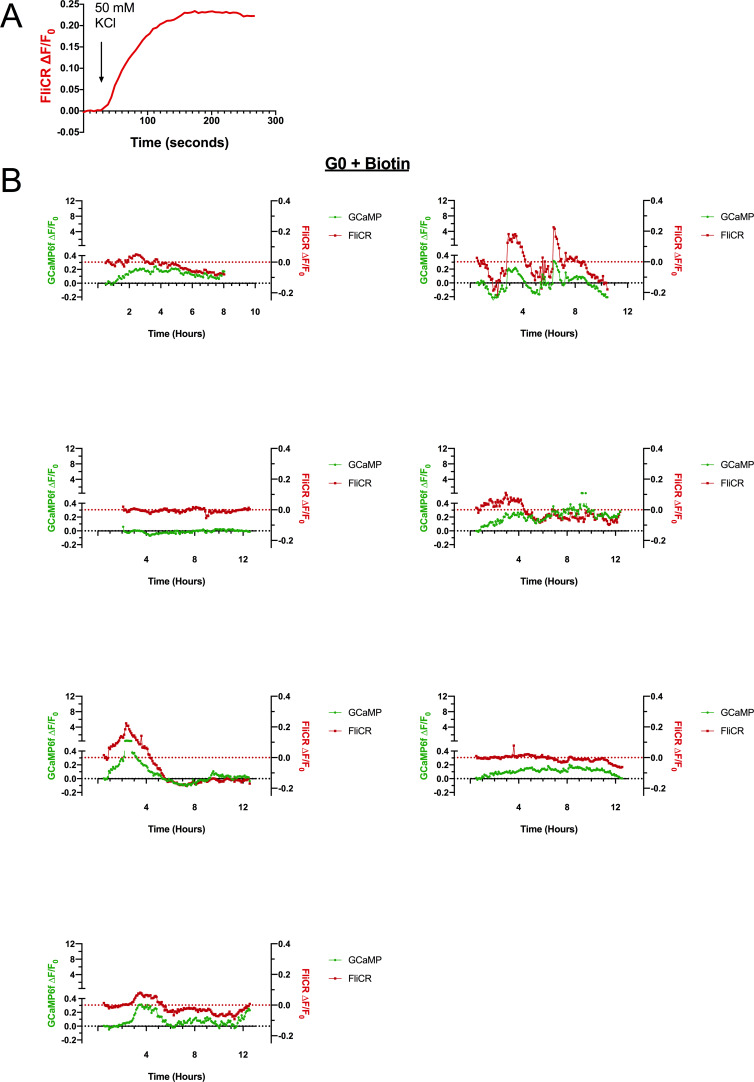
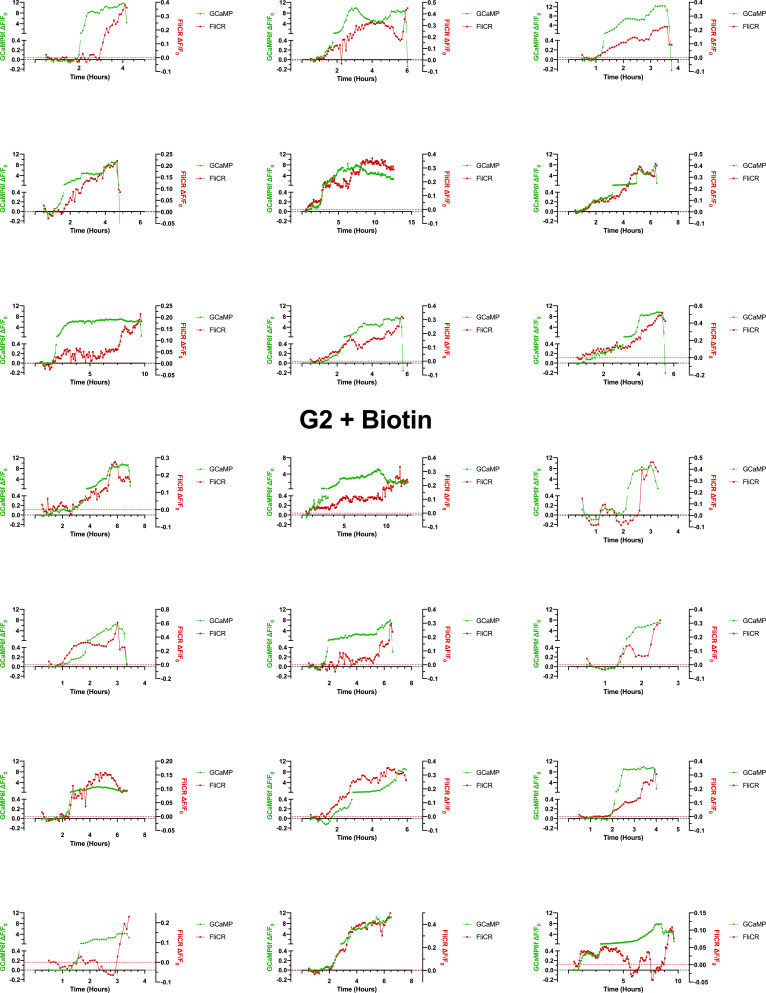
(a) Schematic of the planar lipid bilayer setup showing the sequence of cis buffer perfusions. (b) The APOL1 channel is readily permeable to Na+, but not choline+. In symmetrical KCl solutions Erev was determined as + 1 mV. Then, after cis perfusion with equimolar NaCl buffer (pH 7.2, horizontal bar) there was only a slight change in Erev (Erev = −2 mV; 4 mV scale). In contrast, Erev increased to + 60 mV after exchanging the cis solution for chamber buffer containing equimolar choline chloride. Substituting into the Goldman-Hodgkin-Katz equation (assuming zero permeability to chloride) gives K:Na and K:choline permeability ratios of 1.0:1.1 and 1.0:0.1 respectively. There are two breaks in the record (indicated by //), during which the perfuser was recharged with the appropriate solution. (c) The cytotoxicity of the RRVs in RUSH transfected HEK293 cells is significantly reduced by lowering extracellular Na+ from 150 mM to 85 mM. The rescue from cytotoxicity was indistinguishable between replacement with either K+ or choline+ (n = 9). (d) RRV cytotoxicity was reduced by lowering extracellular Ca2+ from 1.8 mM to 0.45 or 0.1125 mM (n = 12). (e) Reduction of both extracellular Ca2+ and Na+ (replaced by choline+) has an additive effect in lowering RRV cytotoxicity, as seen by further rescue from cell death with 0.45 mM Ca2+ combined with 85 mM Na+ (n = 13). (c–e) Cell death was assayed 12 hr post-biotin treatment with the Promega MultiTox fluorescent assay. Two-way ANOVAs with multiple comparisons were performed. (f) RRV mediated cytotoxicity is driven by the concurrent influx of both Ca2+ and Na+. CHO cells were co-transfected with either RUSH-G0 or G2, GCaMP6f, and the membrane voltage sensor FliCR. G2 cells exhibiting the established phenotype of Ca2+ influx followed by cell swelling were analyzed for changes in membrane voltage (used as a surrogate for the influx of Na+) (n = 21). G0 cells treated with biotin were analyzed for comparison (n = 7). Blue boxes and arrows indicate when the sustained increase in Ca2+ initiates. The representative cells from this figure can be viewed in Figure 6—video 1.