Figure 2, Key Figure. Complement inhibitory proteins and their potential roles in the infection route.
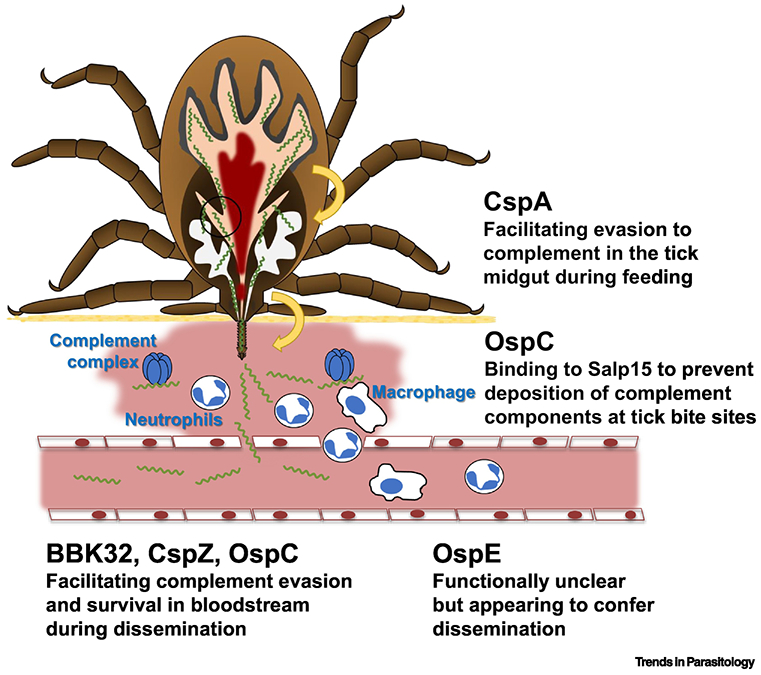
When ticks feed on hosts, B. burgdorferi s.s. produce CspA to facilitate spirochete escape from complement-mediated killing in the blood meal. After transmission to a host, the tick salivary protein, Salp15, binds to OspC on the spirochete surface to prevent opsonophagocytosis at tick bite sites. Additionally, B. burgdorferi s.s. produces OspC, BBK32, and CspZ to promote complement evasion and bloodstream survival of spirochetes. The cell types and complement complex have been indicated on the figure. Though the function of OspE during infection remains unclear, the current evidence supports that this protein may confer spirochete dissemination in vertebrate animals (Part of the figure is adapted from [27]).
