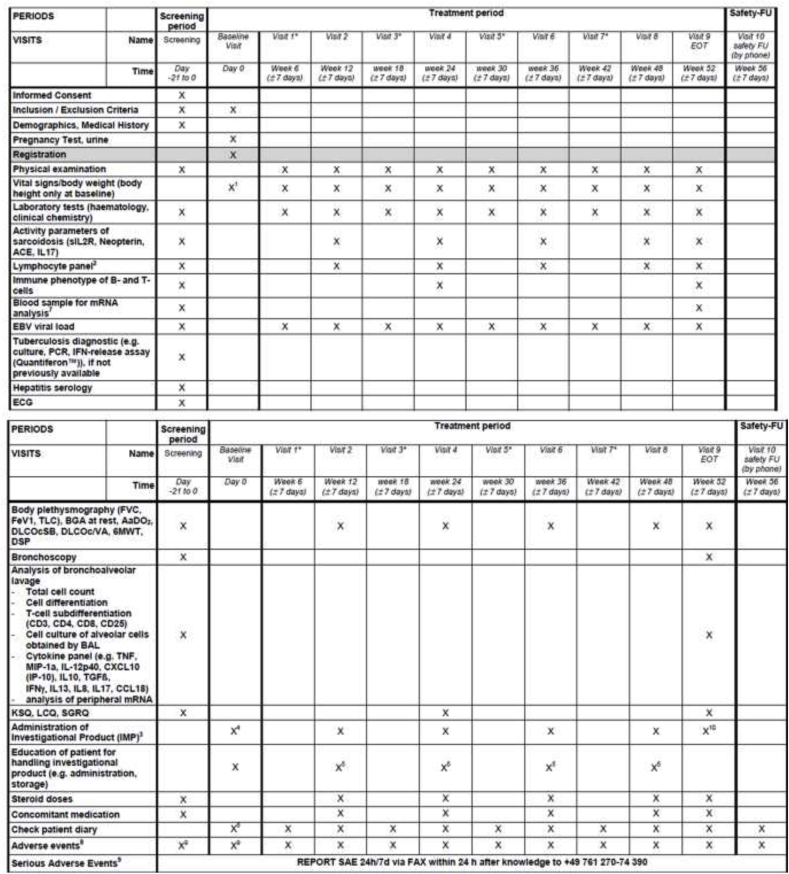
Fig. 2.
Visit schedule and assessment
Fig. 2 highlights the visit schedule for all patients including the screening period, treatment period and follow-up visit. Footnotes are the following:
* visits 1, 3, 5 and 7 are required for part I- group, for part II-group the necessity for these visits will be determined by results from part I-group and advises of the DMC.
1 only at baseline inclusive height
2 CD4, CD8, B and NK cell numbers
3 125 mg/ml syringe weekly; sc injection at clinic and dispensing of investigational product for administration at home (once weekly)
4 administration of first dose of trial medication and subsequent observation for at least 30 min after administration
5 re-education, if needed
6 dispensing of patient diary and education on completion
7 special tubes for mRNA collection
8 documentation period AE/reporting period SAE from 1st application of IMP until 30 days after last application of IMP
9before 1st application of IMP just documentation and reporting of SAE 10 last sc injection at clinic; NO dispensing of investigational product for administration at home (once weekly).