Abstract
Natural killer (NK) cells are pivotal effectors of the innate immunity protecting an individual from microbes. They are the first line of defense against invading viruses, given their substantial ability to directly target infected cells without the need for specific antigen presentation. By establishing cellular networks with a variety of cell types such as dendritic cells, NK cells can also amplify and modulate antiviral adaptive immune responses. In this review, we will examine the role of NK cells in SARS-COV2 infections causing the ongoing COVID19 pandemic, keeping in mind the controversial role of NK cells specifically in viral respiratory infections and in inflammatory-driven lung damage. We discuss lessons learnt from previous coronavirus outbreaks in humans (caused by SARS-CoV-1 and MERS-COV).
Keywords: NK cells, COVID-19, Viral infection, Lung inflammation
1. Introduction
Coronavirus disease 2019 (COVID-19) is a new viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; formerly designated as 2019-nCoV), a novel betacoronavirus firstly identified during a burst of respiratory illness cases in Wuhan City, Hubei Province, China (Li et al., 2020; Zhu et al., 2020; Huang et al., 2020; Zhou et al., 2020b). In a few weeks the disease became a pandemic with 5,593,631 cases and 353,334 confirmed deaths reported as of May 28, (WHO, 2020). A wealth of recent data highlights the disregulated immune response and its inflammatory component as the main casue of morbidity and mortality (Blanco-Melo et al., 2020; Bost et al., 2020; Chen et al., 2020; Diao et al., 2020; Giamarellos-Bourboulis et al., 2020; Liu et al., 2020; Ong et al., 2020; Vardhana and Wolchok, 2020; Zhang et al., 2020; Zhou et al., 2020b; Perini et al., 2020), undescoring the need of a better comprehension of the early events that shape the virus-host reaction in COVID-19.
In ths scenario, it is worth recalling that the components of the innate immune system act as first responder for the detection and clearance of viral infections. Innate immune cells secrete proinflammatory cytokines which inhibit viral replication, stimulate the adaptive immune response, and recruit other immune cells to the site of infection. The implementation of an efficient immune response is a crucial aspect in the control and clearance of virally infected cells. Indeed, innate and adaptive immune responses cooperate to protect the host against microbial infections (Jost and Altfeld, 2013; Vivier et al., 2011; Kumar et al., 2011).
NK cells are innate immune cells whose function is critical in the first-line of defense against against viral, bacterial and parasitic infection (Kumar et al., 2011) as well as in tumor surveillance (Vitale et al., 1992; Zamai et al., 2007), and their functional exhaustion has been correlated to disease progression (Zhang et al., 2019). Furthermore, NK cells are considered a pivotal player in integating innate and adaptive immune reponses (Vivier et al., 2011; Marcenaro et al., 2011). For these reasons, NK cells have been extensively studied in different setting of infectious diseases that have represented, so far, major health issues worldwide such as HBV, HCV and HIV (Rehermann, 2013, Njiomegnie et al., 2020; Lucar et al., 2019; Vitale et al., 2003).
COVID-19 has spread rapidly throughout the globe and is one of the hardest challenge that modern science has to face. At the moment of writing this manuscript, a growing body of data are emerging on variation in the number and function of NK cells during SARS-CoV-2 infection, correlating it with the severity of clinical presentation and outcome (Zheng et al., 2020; Wen et al., 2020; Wilk et al., 2020). However, the functional implications of this observation need to be elucidated.
NK cells are activated by a plethora of cytokines including IL-2, IL-12, IL-15 and type I INF (Ponti et al., 2002a, Ponti et al., 2002b; Vitale et al., 2002; Ponti et al., 2002a, Ponti et al., 2002b; Mirandola et al., 2007; Vitale et al., 2001; Rodella et al., 2001); once triggered, they produce several chemokines such as CCL3/MIP1α, CCL4/MIP1β CCL5/RANTES, and cytokines such as interferon-γ (IFN-γ), tumor necrosis factor (TNF), and granulocyte/macrophage colony-stimulating factor (GM-CSF) (Mirandola et al., 2004). These soluble factors play not only an important regulatory role in hematopoiesis, but also contribute to either priming or taking part to the activation of cellular networks.
In fact, it has been shown that NK cells are engaged in an active and bi-directional cross-talk with autologous dendritic cells (DCs) through a process that requires both NK-cell –DC-cell interactions and secretion of specific cytokines (Jost and Altfeld, 2013; Moretta, 2002; Ferlazzo and Moretta, 2014). Furthermore, monocytes/macrophages and neutrophils have been shown to regulate the recruitment and the activation of NK cells, which, in turn, can eliminate over-stimulated macrophages (Wałajtys-Rode and Dzik, 2017; Molgora et al., 2018). This “mènage à trois” involves direct reciprocal interactions as well as positive amplification loops mediated by cell-derived cytokines, with the aim of inducing IFN-γ production by NK cells (Wałajtys-Rode and Dzik, 2017; Molgora et al., 2018).
The final outcome of these synergic interactions is the coordination and optimization of both innate and adaptive immunity in response to inflammatory stimuli such as viral infections at tissue sites (Moretta, 2002; Moretta et al., 2001; Lugli et al., 2014). In this highly dynamic scenario, NK cells likely configure as important players in determining the quality of the immune responses in COVID-19 patients, critically balancing the direct response to the virus – by eliminating infected cells – and the systemic inflammatory response – by killing DC, monoctyes and T-cells (Chen et al., 2020; Liu et al., 2020; Sun et al., 2020). The loss of this balance appears to be critical during COVID-19 since the overactive cytokine response that typifies the more severe cases rapidly leads to increased risk of vascular hyperpermeability, multiorgan failure, and eventually death (Jose and Manuel, 2020).
2. NK cells as defence against viruses
NK cells are known to be an efficient protective shield against virus infections. First experimental evidence emerged in the late 1980s reporting severe and recurrent herpes virus infections in a young patient with NK cell deficiency (Biron et al., 1989). The fact that NK cells do not need a prior antigen sensitization makes them ready to fight against pathogens starting from the early phases of innate immune responses through several effector functions controlled by a dynamic balance between inhibitory and activating NK cell receptors (NKRs) (Moretta et al., 2001).
Indeed, NK cells are able to lyse “non-self” cellular targets while sparing normal cells that express adequate levels of “self” major histocompatibility complex of class I (MHC-I) molecules. This cytolytic function is regulated by a heterogeneous family of inhibitory NKRs (iNKRs) that bind specifically to either classical or non-classical human leukocyte antigen (HLA) alleles (Orr and Lanier, 2010). Diminution or absence of expression of HLA-I molecules on the surface of virally infected cells results in reduced engagement of iNKRs which, in turns, allow a large group of activating NKRs (aNKRs) to trigger cytotoxicity.
The “on signal” exerted by aNKRs to trigger NK cell killing depends on the induced expression of putative ligands for activating receptors on virally infected target cells (Orr and Lanier, 2010). The recognition of these specific ligands is required for the engagement of aNKR-mediated downstream pathways associated with the NK cell release of lytic granules (Orr and Lanier, 2010). The absence of NK cells results in a significant increased susceptibility to infection (Ashkar and Rosenthal, 2003; Thapa et al., 2007), increasing viral titers and mortality of HSV-2 infected mice. As a critical component of the innate immune response, NK cells act inducing the cytolysis of infected cells (Vivier et al., 2008; Zamai et al., 2007, 2009) and the release of inflammatory cytokines as INF-γ (Orange et al., 1995; Thapa et al., 2007).
Although NK cells are primarily activated by IL-15 released from DCs upon an inflammatory stimulus such as type I-INFs (Lucas et al., 2007; Baranek et al., 2012), other adoptive transfer strategies have been proposed. It has been shown that IFN receptors are not required on NK cells for their activation in the context of Murine Citomegalovirus infection (Guan et al., 2014). Moreover, it has been demonstrated that NK cells may be primed to produce IFN-γ by monocytes via IL-8. Indeed, the innate NK response may be dependent from monocytes, as suggested by the fact that CCR2−/− mouse model, having deficient inflammatory monocyte recruitment, display a significant decrease of IFN-γ production by NK cells (Iijima et al., 2011). The central role of monocytes in NK activation is well described for example in HCV infection, in which in-vitro depletion of inflammatory monocytes from human PBMCs suppressed NK cell responses (Zhang et al., 2013; Serti et al., 2014).
2.1. NK cells in respiratory infections
Extensive evidence exists that early innate functions of NK cells are essential and beneficial in immune defense against respiratory viral infections. These activities include antiviral cytokine production (e.g.,IFN-γ) and lysis of virus-infected cells. At low to intermediate inoculum doses of respiratory syncytial virus (RSV), Sendai virus (parainfluenza virus), and influenza A virus (IAV in mice and hamsters, the activities of NK cells can reduce viral burden and protect from fatal disease (Waggoner et al., 2016; Cong and Wei, 2019).
In the course of an experimental influenza infection of pigs, type I IFN is detected in the bronchoalveolar secretions together with TNF-α, and IL-1 and IL−6. The IFN response starts within 12 h post inoculation, peaks within 18–24 h along with maximal viral replication. In addition, the level of lung pro-inflammatory cytokines correlates with the intensity of clinical signs and neuthrophil infiltrate in the bronchoalveolar lavage fluid (Charley et al., 2006).
The relative contributions of resident vs. circulating NK cells specifically recruited into the lung to pathogen clearance remain undefined.
Nevertheless, the anergic status of human-lung-resident NK cells during homeostasis (Marquardt et al., 2017) suggests that persistence of highly active NK cells in the lung may be more harmful than beneficial, potentially worsening organ injury. Of note, NK cells can potentiate lung injury, reducing survival of mice during respiratory infections that are characterized by higher titers of virus and strong inflammatory responses. A heightened NK-cell activity, resulting in increased IFN-γ production, serves to exhacerbate lung inflammation during both IAV and RSV infections (Cong and Wei, 2019; Abdul-Careem et al., 2012). Moreover, elevated IL-2 and IL-18 amplify this detrimental process, favouring interstitial pneumonia (Okamoto et al., 2002; McKinstry et al., 2019). Irreversible lung damage by NK cells may be more than just an unfortunate side effect of IFN-γ production, as the robust cytolytic elimination of virus-infected airway epithelial cells by NK cells is a critical antiviral function that may exceed the functional and regenerative capacity of the lung.
2.2. NK cells in non-COVID-19 coronavirus infections
As discussed above, NK cells are involved in viral infection control in animal models and humans (Waggoner et al., 2016). However, to the best of our knowledge, the interplay between NK cells and SARS has been scarcely described.
Hua et al. proved that nasal inoculation of Murine Hepatitis Virus Strain 1 (MHV-1, capable to reproduce a clinical model of SARS in Mice), primes NK activation and increase pulmonary recruitment of Ly6C + inflammatory monocytes via type I INF signaling, generating a innate immune-mediated control toward second coronavirus infection (Hua et al., 2018).
NK cell cytotoxicity is regulated by a multitude of receptors including CD158b, which binds to MHC-I expressed on target cells (Orr and Lanier, 2010; Jost and Altfeld, 2013). Xia et al. (2004) reported that, in patients with SARS, the total number of NK cells, as well as the total number and percentage of CD158b+ NK cells, were significantly lower than patients with interstitial pneumonia caused by Mycoplasma pneumoniae and than control subjects. The number of NK cells and CD158b+ NK cells remained low for the entire disease course and began to recover after the 40th day of the disease. In severe SARS cases, all three parameters were significantly lower than those in mild cases of SARS. No significant differences were found between the group with mild SARS and that with M. pneumoniae infection (Xia et al., 2004).
How the SARS virus alters the number and function of NK cells needs to be elucidated. The mechanism of CD158b down-regulation in patients with SARS is still under investigation, and, according to Xia et al. (2004), two possible mechanisms might underlie under this process: (i) CD158b is detached from the NK surface and becomes soluble in the serum; and/or (ii) the expression of CD158b is down-regulated at the transcriptional or translational level. Concerning the reduction in total NK number, possible explations may be: (i) NK cytolysis after killing the infected target cells; and/or (ii) redistribution to targeted organs (e.g., the lung).
2.3. NK cells in COVID-19
Several evidences support the fact that lymphopenia is associated with severe clinical presentation of COVID-19. Specifically, T cell- and CD8+ T cell-count were reduced in COVID-19 patients as compared to non-infected cases and, among COVID-19 patients, severe cases presented significantly lower counts as compared to mild cases (Zheng et al., 2020, Chen et al., 2020, Jiang et al., 2020 Wang et al., 2020; Wilk et al., 2020). Similarly, NK cell count reduces remarkably during Sars-Cov-2 infection, predominantely in critically ill patients (Wen et al., 2020; Zheng et al., 2020; Giamarellos-Bourboulis et al., 2020). This is is consistent with previous findings in SARS as outlined above (Xia et al., 2004) and it is conceivable that this finding is due to NK sequestration into target organs, e.g. the lung. However, it is unclear at this time if this decrease is due to NK cell redistribution in infected sites or cell death.
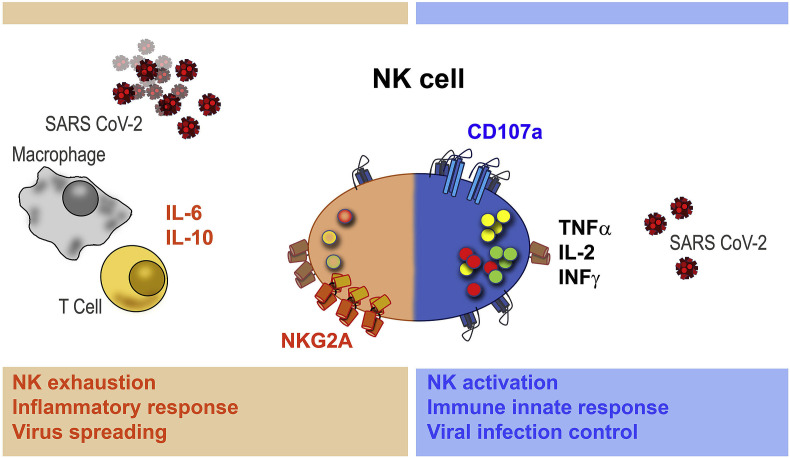
In addition, a very intersting mechanism of T and NK cell exhaustion has been hypothesized by Zheng et al. (2020). In their work, the authors observe that the NK group 2 member A (NKG2A) receptor, which transduces inhibitory signalling and suppresses T-cell and NK cytokine secretion and cytotoxic function, is overexpressed in COVID-19 patients as compared to healthy controls, while the percentage of T and NK cells expressing the activation markers CD107a, IFNɣ, IL-2, and TNFɑ was significanly lower (Zheng et al., 2020). Taken together, these data indicate that patients with severe COVID-19 have a severely compromised innate immune response likely due to a functional exhaustion of peripheral CD8+ T and NK cells (Fig. 1 ).
Fig. 1.
Hypothesized double-sided mechanism of Sars-Cov-2 and NK cells interaction. In case of effective immune innate response, NK cells express the activation marker CD107a and release IFNɣ, IL-2, and TNFɑ (right side). In case of exhaustion, NK cells overexpress the inhibitory NKG2A receptor, which suppress T-cell and NK cytotoxic function, favoring a pro-inflammatory condition (left side).
Loss of NK cell effector functions is the most prominent immunological feature of the macrophage activation syndrome (also reffered to as hemophagocytic lymphohistiocytosis, HLH), a condition that can be triggered by infections and that closely resembles the “hyperferritinemic syndrome” that Shoenfeld et al. compare to Sars-CoV-2-related cytokine storm (Shoenfeld, 2020). Similarly to what happens in HLH, local and systemic inflammation contributes to reduce NK cell effector functions; specifically, elevated IL-6 and IL-10 levels (as the ones observed in COVID-19) are capable to inhibit NK cytotoxic activity as the expression of PERF and granzyme B). Moreover, IL-6 may further impair NK activity by reducing the expression of NKG2D, important in the killing of infected cells (Osman et al., 2020).
Xiong et al. showed that several upregulated genes in PBMCs from COVID-19 patients are involved in the apoptosis pathways, suggesting lymphopenia may be due to SARS-CoV-2-mediated apoptosis, supporting therefore the cell-death hypothesis (Xiong et al., 2020).
By contrast, in favour to the target-site sequestration mechanism, scRNA-seq analysis of bronchoalveolar lavage fluid (BALF) samples from COVID-19 patients (three severe and three mild cases) allowed detection of higher amounts of NK cells in COVID-19 patients as compared to controls, suggesting NK cell trafficking into the lungs (Liao et al., 2020). Finally, bulk RNA-seq on BALF from eight COVID-19 cases, found a significant decrease in resting NK cells in COVID-19 patients compared to healthy controls, but no changes in the number of activated NK cells (Zhou et al., 2020a).
The discrepancies between these two studies could be explained by the fact that in the cohort analyzed by Zhou et al. (2020a), the time of sampling was closer to the day of symptoms onset, suggesting that the trafficking of NK cells into the lungs might occur in a time-dependent fashion, preferenatially in the stages of infection.
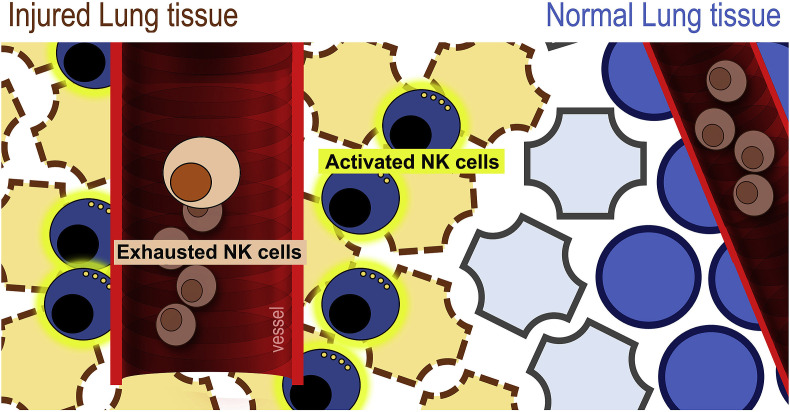
Taken together these data, although preliminary, suggest that upon SARS-CoV-2 infection, NK cells exit the peripheral blood, traffick into to the lung where potentially contribuite to local inflammation and injury. By constrast, NK cells that remain in the circulation display an exhausted phenotype that facilitate virus spread to other organs (Fig. 2 ).
Fig. 2.
Hypothesized opposite behavior of circulating vs. lung-resident NK cells during COVID-19: NK cells that remain in the circulation display an exhausted phenotype that facilitate virus spread while NK cells that exit blood and traffic into the lung contribute to local inflammation and tissue damage.
The rationale of using NK cells and/or NK cell modulation in COVID 19 disease has to be based on the timing of therapeutic “fine tuning” of NK cells, which would likely determine the balance between their beneficial antiviral and detrimental pathologic action. During Sendai parainfluenza virus infection in mice, early infusion of NK cells partially controlled low-dose inoculum infection, whereas therapy initiation at a later stage of infection increased viral replication and associated morbidity (Mostafa et al., 2018). Similarly, patient-specific factors are likely to impact the efficacy of NK-cell based therapeutics, as severe SARS-CoV-2 infection is associated with hypoxia and elevated IL-6 (Chen et al., 2020), which can significantly impair the function of NK cells (Cifaldi et al., 2015). The potential role of elevated IL-6 in poor outcome of SARS-CoV-2 infections has even led to clinical trials investigating the utility of drugs that inhibit IL-6 (e.g., tocilizumab; https://clinicaltrials.gov/ct2/show/NCT04335071) or JAK signaling (e.g., tofacitinib; https://clinicaltrials.gov/ct2/show/NCT04332042) in the treatment of critical illness in SARS-CoV-2 patients. Some preliminary data in this regard is already available in the published literature (Luo et al., 2020; Di Giambenedetto et al., 2020; Xu et al., 2020; Capra et al., 2020; Morena et al., 2020; Campochiaro et al., 2020). Of note, tofacitinib and related JAK-inhibitors can reduce the number and function of NK cells, highlighting how clinical benefit of such drugs could stem, in part, from depletion of potentially pathogenic NK cells.
3. Conclusions
COVID-19 is characterized by substantial components of what is considered to be a special dysregulated and imbalanced innate immune response to SARS-CoV-2 infection. Of note, we do not really know at the moment the features of a proficient and effective innate immune response towrd SARS-CoV-2. A likely solution to this impelling problem is to assess patient populations that, up until now, have been relatively overlooked. In fact, the vast majority of COVID-19 studies have focused on patients with serious/severe disease.
A critical part of our understanding of SARS-CoV-2 infection, and potentially useful to identify a coordinated, successful immune response, would be to assess COVID-19 patients with mild disease, not requiring hospitalization. A longitudinal study comparing the immune responses of hospitalized versus non-hospitalized patients would be pivotal to delineate this relevant insight. Such studies will provide a path as to how we need to modulate the immune response for maximum benefit and to improve patient recovery. Undestanding in more detail the interface between innate immunity, adaptive immuty and SARS-CoV-2 will be instrumetal in increasing favourable patient outcome and helping to design future immune interventions aimed to modulatee disease impact and/or to prevent disease occurrence (such as vaccines).
Authors’ contribution
P.M. M. Vitale, M. Vaccarezza: manuscript conceptualization and writing (review & editing).E.M., C.C.: manuscript conceptualization and writing (original draft), data curation. G.P., V.P.: data curation
Declaration of competing interest
None.
Acknowledgements
This work was supported by: Fondi Locali per la Ricerca 2019 “Quota Prodotto della Ricerca” to E.M., C.C., and M. Vitale.
References
- Abdul-Careem M.F., Mian M.F., Yue G., Gillgrass A., Chenoweth M.J., Barra N.G., Chew M.V., Chan T., Al-Garawi A.A., Jordana M., Ashkar A.A. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. J. Infect. Dis. 2012;206:167–177. doi: 10.1093/infdis/jis340. [DOI] [PubMed] [Google Scholar]
- Ashkar A.A., Rosenthal K.L. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 2003;77:10168–10171. doi: 10.1128/JVI.77.18.10168-10171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranek T., Manh T.P., Alexandre Y., Maqbool M.A., Cabeza J.Z., Tomasello E., Crozat K., Bessou G., Zucchini N., Robbins S.H., et al. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe. 2012;12:571–584. doi: 10.1016/j.chom.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Biron C.A., Byron K.S., Sullivan J.L. Severe herpes virus infection in an adolescent without natural killer cells. N. Engl. J. Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Blanco-Melo D., Nilsson-Payant B.E., Liu W.C., Uhl S., Hoagland D., Møller R., Jordan T.X., Oishi K., Panis M., Sachs D., Wang T.T., Schwartz R.E., Lim J.K., Albrecht R.A., tenOever B.r. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost P., Giladi A., Liu Y., Bendjelal Y., Xu G., David E., Blecher-Gonen R., Cohen M., Medaglia C., Li H., Deczkowska A., Zhang S., Schwikowski B., Zhang Z., Amit I. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020;(S0092–8674(20)) doi: 10.1016/j.cell.2020.05.006. 30568–7 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro C., Della –Torre E., Cavalli G., De Luca G., Ripa M., Boffini M., Tomelleri A., Baldissera E., Rovere-Querini R., Ruggeri P., Monti G., De Cobelli F., Zangrillo A., Tresoldi M., Castagna A., Dagna L. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur. J. Intern. Med. Jun 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M.P., Cossi S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. Jun 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charley B., Riffault S., Van Reeth K. Porcine innate and adaptative immune responses to influenza and coronavirus infections. Ann. N. Y. Acad. Sci. 2006;1081:130–136. doi: 10.1196/annals.1373.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifaldi L., Prencipe G., Caiello I., Bracaglia C., Locatelli F., De Benedetti F., Strippoli R. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheum. 2015;67:3037–3046. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- Cong J., Wei H. Natural killer cells in the lungs. Front. Immunol. 2019;10:1416. doi: 10.3389/fimmu.2019.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E., Zileri Dal Verme L., Bernabei R., Tamburrini E., Cauda R., Gasbarrini A. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J. Med. Virol. Apr 16 2020 doi: 10.1002/jmv.25897. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo G., Moretta L. Dendritic cell editing by natural killer cells. Crit. Rev. Oncog. 2014;19:67–75. doi: 10.1615/critrevoncog.2014010827. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.E., Katsaounou P., Ntaganou M., Kyriakopoulou M., Dimopoulos G., Koutsodimitropoulos I., Velissaris D., Koufargyris P., Karageorgos A., Katrini K., Lekakis V., Lupse M., Kotsaki A., Renieris G., Theodoulou D., Panou V., Koukaki E., Koulouris N., Gogos C., Koutsoukou A. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J., Miah S.M., Wilson Z.S., Erick T.K., Banh C., Brossay L. Role of type I interferon receptor signaling on NK cell development and functions. PloS One. 2014;9 doi: 10.1371/journal.pone.0111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X., Vijay R., Channappanavar R., Athmer J., Meyerholz D.K., Pagedar N., Tilley S., Perlman S. Nasal priming by a murine coronavirus provides protective immunity against lethal heterologous virus pneumonia. JCI Insight. 2018;3(11) doi: 10.1172/jci.insight.99025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N., Mattei L.M., Iwasaki A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc. Natl. Acad. Sci. U.S.A. 2011;108:284–289. doi: 10.1073/pnas.1005201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Guo Y., Luo Q., Huang Z., Zhao R., Liu S., Le A., Li J., Wan L. T cell subset counts in peripheral blood can be used as discriminatory biomarkers for diagnosis and severity prediction of COVID-19. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa252. May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020;27 doi: 10.1016/S2213-2600(20)30216-2. S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost S., Altfeld M. Control of human viral infections by natural killer cells. Annu. Rev. Immunol. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. May 12 2020 doi: 10.1038/s41591-020-0901-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Liu J., Li S., Zhiu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., et al. Longitudinal characteristrics of lymphocyte responses and cytokine profiles in the peruipheral blood of SARS-CoV-2 infected patients. Ebiomedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucar O., Reeves R.K., Jost S. A natural impact: NK cells at the intersection of cancer and HIV disease. Front. Immunol. 2019;10:1850. doi: 10.3389/fimmu.2019.01850. Aug 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli E., Marcenaro E., Mavilio D. NK cell subset redistribution during the course of viral infections. Front. Immunol. 2014;5:390. doi: 10.3389/fimmu.2014.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcenaro E., Carlomagno S., Pesce S., Moretta A., Sivori S. Bridging innate NK cell functions with adaptive immunity. Adv. Exp. Med. Biol. 2011;780:45–55. doi: 10.1007/978-1-4419-5632-3_5. [DOI] [PubMed] [Google Scholar]
- Marquardt N., Kekäläinen E., Chen P., Kvedaraite E., Wilson J.N., Ivarsson M.A., Mjösberg J., Berglin L., Säfholm J., Manson M.L., et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69−CD56dim cells. J. Allergy Clin. Immunol. 2017;139:1321–1330. doi: 10.1016/j.jaci.2016.07.043. [DOI] [PubMed] [Google Scholar]
- McKinstry K.K., Alam F., Flores-Malavet V., Nagy M.Z., Sell S., Cooper A.M., Swain S.L., Strutt T.M. Memory CD4 T cell-derived IL-2 synergizes with viral infection to exacerbate lung inflammation. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirandola P., Gobbi G., Sponzilli I., Pambianco M., Malinverno C., Cacchioli A., De Panfilis G., Vitale M. Exogenous hydrogen sulfide induces functional inhibition and cell death of cytotoxic lymphocytes subsets. J. Cell. Physiol. 2007;213(3):826–833. doi: 10.1002/jcp.21151. [DOI] [PubMed] [Google Scholar]
- Mirandola P., Ponti C., Gobbi G., Sponzilli I., Vaccarezza M., Cocco L., Zauli G., Secchiero P., Manzoli F.A., Vitale M. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Bloob. 2004;104(8):2418–2424. doi: 10.1182/blood-2004-04-1294. 15. [DOI] [PubMed] [Google Scholar]
- Molgora M., Supino D., Mavilio D., Santoni A., Moretta L., Mantovani A., Garlanda C. The yin-yang of the interaction between myelomonocytic cells and NK cells. Scand. J. Immunol. 2018;88 doi: 10.1111/sji.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morena V., Milazzo L., Oreni L., Bestetti G., Fossati T., Bassoli C., Torre A., Cossu M.V., Minari C., Ballone E., Perotti A., Mileto D., Niero F., Merli S., Foschi A., Vimercato S., Rizzardini G., Sollima S., Bradanin L., Galimberti L., Colombo R., Micheli V., Negri C., Ridolfo A.L., Meroni L., Galli M., Antinori S., Corbellino M. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur. J. Intern. Med. 2020;76:36–42. doi: 10.1016/j.ejim.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Bottino C., Vitale M., Pende D., Cantoni C., Mingari M.C., Biassoni R., Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- Moretta A. Natural killer cells and dendritic cells: rendez-vous in abused tissues. Nat. Rev. Immmunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- Mostafa H.H., Vogel P., Srinivasan A., Russell C.J. Dynamics of Sendai virus spread, clearance, and immunotherapeutic efficacy after hematopoietic cell transplant imaged noninvasively in mice. J. Virol. 2018;92:e01705–e01717. doi: 10.1128/JVI.01705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njiomegnie G.F., Read S.A., Fewings N., George J., McKay F., Ahlenstiel G. Immunomodulation of the natural killer cell phenotype and response during HCV infection. J. Clin. Med. 2020;9(4):1030. doi: 10.3390/jcm9041030. 2020 Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M., Kato S., Oizumi K., Kinoshita M., Inoue Y., Hoshino K., Akira S., McKenzie A.N., Young H.A., Hoshino T. Interleukin 18 (IL-18) in synergy with IL-2 induces lethal lung injury in mice: a potential role for cytokines, chemokines, and natural killer cells in the pathogenesis of interstitial pneumonia. Blood. 2002;99:1289–1298. doi: 10.1182/blood.v99.4.1289. [DOI] [PubMed] [Google Scholar]
- Ong E.Z., Chan Y.F.Z., Leong W.Y., Lee N.M.Y., Kalimuddin S., Haja Mohideen S.M., Chan K.S., Tan A.T., Bertoletti A., Ooi E.E., Low J.G.H. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27(6):879–882. doi: 10.1016/j.chom.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J.S., Wang B., Terhorst C., Biron C.A. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J. Exp. Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M.T., Lanier L.L. Natural killer cell education and tolerance. Cell. 2010;142:847–856. doi: 10.1016/j.cell.2010.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman M.S., van Eeden C., Cohen Tervaert J.W. Fatal COVID-19 infections: is NK cell dysfunction a link with autoimmune HLH? Autoimmun Rev. 2020;3:102561. doi: 10.1016/j.autrev.2020.102561. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti C., Gibellini D., Boin F., Melloni E., Manzoli F.A., Cocco L., Zauli G., Vitale M. Role of CREB transcription factor in c-fos activation in natural killer cells. Eur. J. Immunol. 2002;32(12):3358–3365. doi: 10.1002/1521-4141(200212)32:12<3358::AID-IMMU3358>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Perini P., Nabulsi B., Massoni C.B., Azzarone M., Freyrie A. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395(10236):1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti C., Falconi M., Billi A.M., Faenza I., Castorina S., Caimi L., Cacchioli A., Cocco L., Vitale M. IL-12 and IL-15 induce activation of nuclear PLCbeta in human natural killer cells. Int. J. Oncol. 2002;20(1):149–153. [PubMed] [Google Scholar]
- Rehermann B. Pathogenesis of chronic viral hepatitis: differential roles of T cells and NK cells. Nat. Med. 2013;19:859–868. doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodella L., Zamai L., Rezzani R., Artico M., Peri G., Falconi M., Facchini A., Pelusi G., Vitale M. Interleukin 2 and interleukin 15 differentially predispose natural killer cells to apoptosis mediated by endothelial and tumour cells. Br. J. Haematol. 2001;115(2):442–450. doi: 10.1046/j.1365-2141.2001.03055.x. 2001 Nov. [DOI] [PubMed] [Google Scholar]
- Serti E., Werner J.M., Chattergoon M., Cox A.L., Lohmann V., Rehermann B. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology. 2014;147:209–220.e3. doi: 10.1053/j.gastro.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. doi: 10.1016/j.autrev.2020.102538. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Dong Y., Wang L., Xie H., Li B., Chang C., Wang F.S. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J. Autoimmun. 2020;24:102473. doi: 10.1016/j.jaut.2020.102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapa M., Kuziel W.A., Carr D.J. Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J. Virol. 2007;81:3704–3713. doi: 10.1128/JVI.02626-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhana S.A., Wolchok J.D. The many faces of the anti-COVID immune reponse. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale M., Caruso A., De Francesco M.A., Rodella L., Bozzo L., Garrafa E., Grassi M., Gobbi G., Cacchioli A., Fiorentini S. HIV-1 matrix protein p17 enhances the proliferative activity of natural killer cells and increases their ability to secrete proinflammatory cytokines. Br. J. Haematol. 2003;120(2):337–343. doi: 10.1046/j.1365-2141.2003.04053.x. 2003 Jan. [DOI] [PubMed] [Google Scholar]
- Vitale M., Bassini A., Secchiero P., Mirandola P., Ponti C., Zamai L., Mariani A.R., Falconi M., Azzali G. NK-active cytokines IL-2, IL-12, and IL-15 selectively modulate specific protein kinase C (PKC) isoforms in primary human NK cells. Anat. Rec. 2002;266(2):87–92. doi: 10.1002/ar.10039. 1. [DOI] [PubMed] [Google Scholar]
- Vitale M., Matteucci A., Manzoli L., Rodella L., Mariani A.R., Zauli G., Falconi M., Billi A.M., Martelli A.M., Gilmour R.S., Cocco L. Interleukin 2 activates nuclear phospholipase Cbeta by mitogen-activated protein kinase-dependent phosphorylation in human natural killer cells. Faseb. J. 2001;15(10):1789–1791. doi: 10.1096/fj.01-0008fje. [DOI] [PubMed] [Google Scholar]
- Vitale M., Zamai L., Neri L.M., Galanzi A., Facchini A., Rana R., Cataldi A., Papa S. The impairment of natural killer function in the healthy aged is due to a postbinding deficient mechanism. Cell. Immunol. 1992;145(1):1–10. doi: 10.1016/0008-8749(92)90307-b. [DOI] [PubMed] [Google Scholar]
- Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L., Yokoyama W.M., Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner S.N., Reighard S.D., Gyurova I.E., Cranert S.A., Mahl S.E., Karmele E.P., McNally J.P., Moran M.T., Brooks T.R., Yaqoob F., Rydyznski C.E. Roles of natural killer cells in antiviral immunity. Curr. Opin. Virol. 2016;16:15–23. doi: 10.1016/j.coviro.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wałajtys-Rode E., Dzik J.M. Monocyte/macrophage: NK cell cooperation-old tools for new functions. Results Probl. Cell Differ. 2017;62:73–145. doi: 10.1007/978-3-319-54090-0_5. [DOI] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221(11):1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., Liu X., Xie L., Li J., Ye J., et al. Immune cell profiling of COVID-19 patients in the recovery stage by singlecell sequencing. Cell Discov. 2020;6:31. doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Who . 2020. Coronavirus Disease (COVID-2019) Situation Reports.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
- Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martinez-Colon G.J., McKechnie J.L., Ivison G.T., Ranganath T., Vergara R., Hollis T., et al. 2020. A single-cell atlas of the peripheral immune response to severe COVID-19. medRxiv Preprint. 2020 Apr 23. (020.04.17.20069930) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C.Q., Xu L.L., Wang Z., Qin Z.Q., Tong Z.H., Huang K.W., Xiao B., Qi M., Jiang B.Z., Wang C., et al. National Research Project for Sars The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome. Am. J. Clin. Pathol. 2004;121:507–511. doi: 10.1309/WPK7Y2XKNF4CBF3R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microb. Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L., Ponti C., Mirandola P., Gobbi G., Papa S., Galeotti L., Cocco L., Vitale M. NK cells and cancer. J. Immunol. 2007;178:4011–4016. doi: 10.4049/jimmunol.178.7.4011. [DOI] [PubMed] [Google Scholar]
- Zamai L., Galeotti L., Del Zotto G., Canonico B., Mirandola P., Papa S. Identification of a NCR+/NKG2D+/LFA-1(low)/CD94(-) immature human NK cell subset. Cytometry. 2009;75:893–901. doi: 10.1002/cyto.a.20789. [DOI] [PubMed] [Google Scholar]
- Zhang S., Saha B., Kodys K., Szabo G. IFN-γ production by human natural killer cells in response to HCV-infected hepatoma cells is dependent on accessory cells. J. Hepatol. 2013;59:442–449. doi: 10.1016/j.jhep.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z., Jia X., Wu M., Shi B., Xu S., Chen J., Wang W., Chen B., Jiang L., Yu S., Lu J., Wang J., Xu M., Yuan Z., Zhang Q., Zhang X., Zhao G., Wang S., Chen S., Lu H. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;May 20 doi: 10.1038/s41586-020-2355-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang X.M., Li S.R., Twelkmeyer T., Wang W.H., Zhang S.Y., Wang S.F., Chen J.Z., Jin X., Wu Y.Z. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat. Commun. 2019;10(1):1507. doi: 10.1038/s41467-019-09212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Guo L., Yang J., Wang C., Jiang S., Yang D., Zhang G., Li H., Chen F., Xu Y., Chen M., Gao Z., Yang J., Dong J., Liu B., Zhang X., Wang W., He K., Jin Q., Li M., Wang J. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020;27(6):883–890. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]