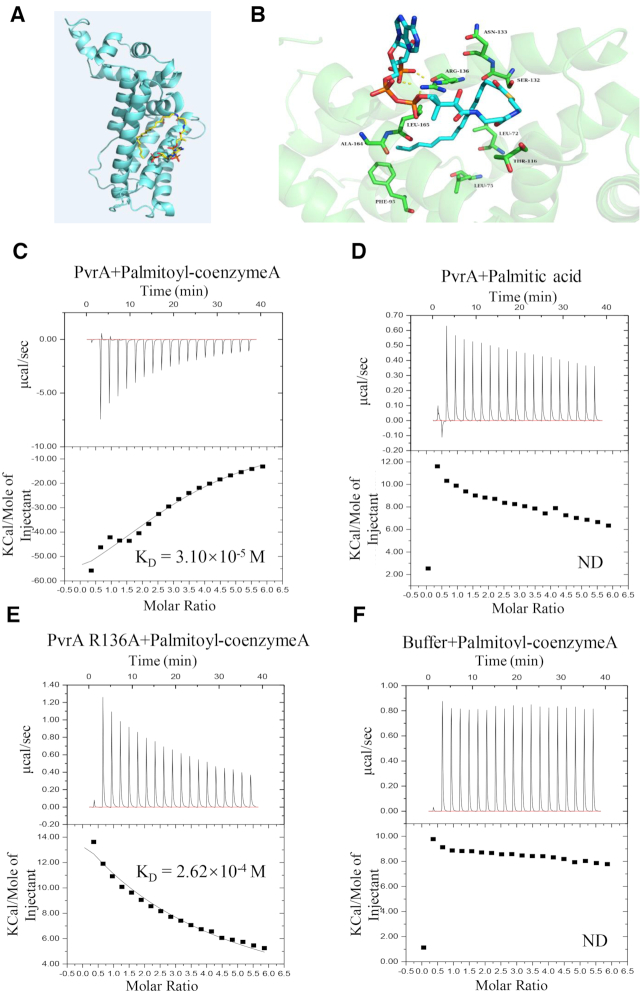
Figure 7.
Interaction between PvrA and palmitoyl-coenzyme A. (A) Model structure of the interaction between palmitoyl-coenzyme A and PvrA by the MetaPocket program. (B) Binding modes of palmitoyl-coenzyme A in the PvrA binding pocket. Dotted lines represent hydrogen-bonds between palmitoyl-coenzyme A and the arginine residue at the 136 position of PvrA. (C–F) Direct binding of palmitoyl-coenzyme A to the soluble PvrA was measured by isothermal titration microcalorimetry (ITC). Isothermal titration microcalorimetry of palmitoyl-coenzyme A (C) or palmitic acid (D) (0.3 mM) binding to 0.03 mM PvrA protein at 25°C. Palmitoyl-coenzyme A exhibited binding affinity of KD = 3.10 × 10−5 M to PvrA; No detectable binding of palmitic acid to PvrA was exhibited. (E) ITC analysis revealed the affinity between PvrA R136A mutant and palmitoyl-coenzyme A was KD = 2.62 × 10−4 M. (F). Interaction between the buffer and palmitoyl-coenzyme A was analysed as negative control. Affinity and molar ratio are indicated. ND: No detectable binding.