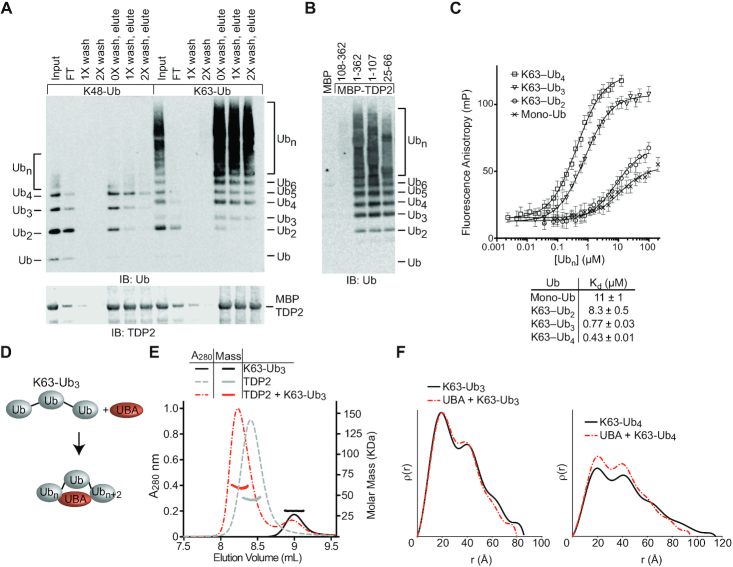
Figure 2.
TDP2-UBA domain binds K63Ub3 with a 1:1 stoichiometry. (A) TDP2 binds K48 and K63 poly-Ub chains. Poly-Ub interaction was assessed in binding reactions containing amylose resin, MBP-TDP2 fusion protein, and poly-Ub chains. Proteins present in the input, flow through (FT), washes, and elution were detected by SDS-PAGE and western blotting. Ubn indicates longer chains that are not resolved in the gel. (B) Domain analysis of TDP2. MBP-TDP2 fusion protein constructs were assayed for binding to K63-Ub chains as in panel A. (C) Fluorescence polarization anisotropy assay. Fluorescein-UBA protein was incubated with increasing amounts of the indicated Ub or K63-Ub chain and the FP value was measured at each concentration. Error bars, s.d. N = 4 replicates. Data were fit to single binding-site model (solid line) to calculate Kd values ± s.e.m. (D) Model for compaction of K63-Ub3 upon binding the UBA domain. (E) Molar mass determination of proteins using size-exclusion chromatography with multi-angle light scattering (SEC-MALS) indicates TDP2 binds Ub3 with a 1:1 stoichiometry. (F) ρ(r) plots calculated from SAXS scattering data of the indicated protein or protein complexes.