Figure 7.
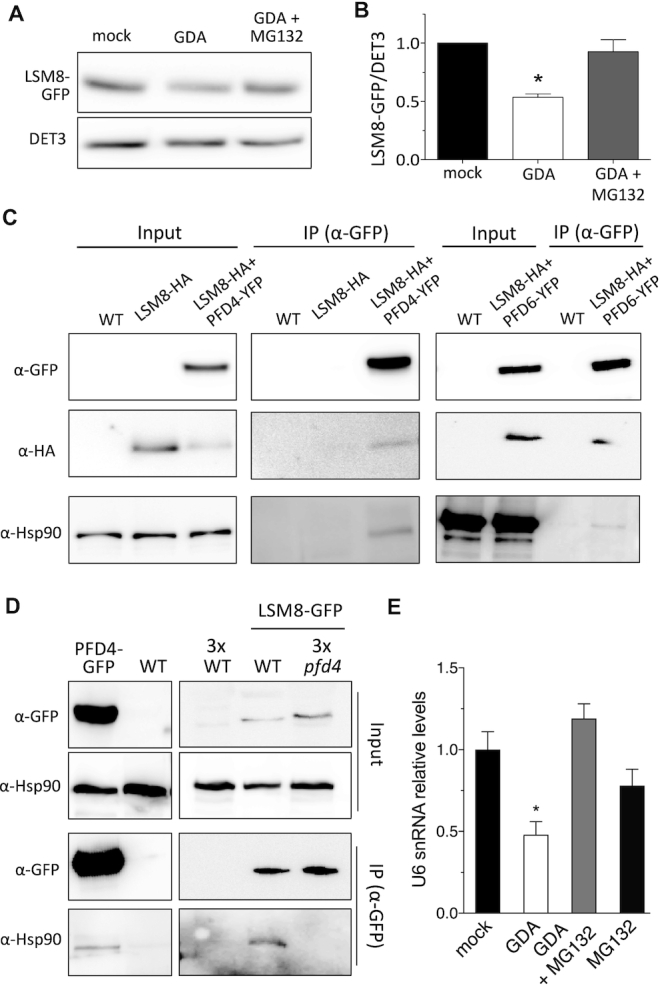
Hsp90 stabilizes LSM8 protein. (A, B) LSM8-GFP protein levels in response to 24 h treatments with 20 μM GDA or with 20 μM GDA + 50 μM MG132. A representative blot is shown in (A). (B) Plot showing the LSM8-GFP levels after normalization to DET3 that is used as loading control. Data are average from two biological replicates. One asterisk represents P < 0.01 in an ANOVA test. (C) Co-immunoprecipitation assay showing the interaction of PFD4-YFP or PFD6-YFP with LSM8-HA and Hsp90. Total proteins were immunoprecipitated with anti-GFP antibody-coated paramagnetic beads from extracts of 7-day-old seedlings. Proteins were detected with anti-GFP, anti-HA and anti-Hsp90 antibodies. (D) Co-immunoprecipitation assay showing that PFD4 mediates the interaction of Hsp90 with LSM8-GFP in Arabidopsis. Total proteins were immunoprecipitated with anti-GFP antibody-coated paramagnetic beads from extracts of 7-day-old seedlings. Proteins were detected with anti-GFP and anti-Hsp90 antibodies. Note that we used three times the amount of protein extract from non-transgenic control and pfd4 seedlings for the immunoprecipitation. (E) Relative U6 snRNA levels in response to treatments with 20 μM GDA, with 20 μM GDA + 50 μM MG132 or with 50 μM MG132. U6 snRNA levels were normalized against PP2AA3 mRNA. Data are average of three biological replicates. Error bars indicate standard error of mean.
