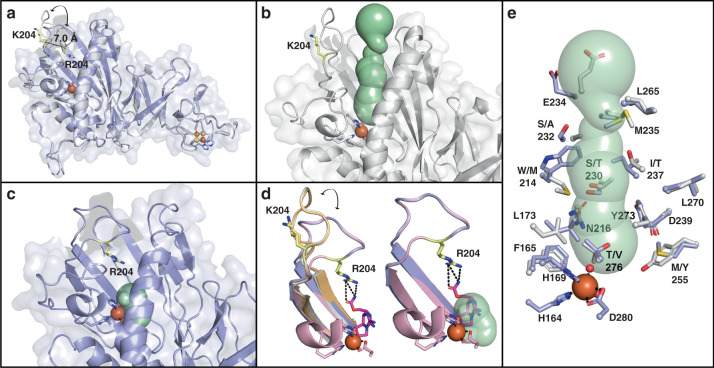
Fig. 5. SxtT and GxtA exhibit different conformations of a loop that appears to gate access to the active site.
a An overlay of the SxtT and GxtA monomers reveals a 7.0 Å difference in the location of the residue at the 204 position. This difference is due to changes in the position of a loop that spans the range 195–215. b The open conformation of the loop in GxtA allows visualization of a tunnel that leads directly from the protein surface to the active site. c The closed orientation of the loop in SxtT restricts calculation of an equivalent tunnel, but instead allows visualization of the active site cavity. d An overlay of all four structures determined in this work reveals that Arg204 in the co-crystal structure assumes a similar position to that seen in the ligand free structure of SxtT. In this orientation, Arg204 interacts with ddSTX. e An overlay of SxtT and GxtA shows differences in the residues that line the putative tunnel to the active site. In all panels, structures are shown for SxtT, GxtA, ddSTX-SxtT, and ddSTX-GxtA in purple, gray, pink, and orange respectively. Tunnels are colored mint, ddSTX is dark pink, and the Arg/Lys204 residue is yellow.