Highlights
-
•
Coronavirus Disease 2019 is a newly discovered viral disease whose knowledge is constantly evolving.
-
•
Spontaneous pneumomediastinum and pneumothorax are rarely found in COVID-19 pneumonia.
-
•
Computed Tomography is the modality of choice in the evaluation of Meckline effect.
Keywords: COVID-19, CT, Spontaneous pneumomediastinum, Loculated pneumothorax
Abbreviations: ARDS, Acute distress respiratory syndrome; ACE2, Angiotensin-converting enzyme 2; COVID-19, Coronavirus Disease 2019; CT, Chest computed tomography; DPP4, Dipeptidyl peptidase 4; GGO, Ground glass opacities; LPNX, Loculated pneumothorax; MERS, Middle East respiratory syndromes; NIV, Non-invasive respiratory support; PNX, Pneumothorax; RT-PCR, Real-time reverse transcriptase polymerase chain reaction; SARS, Severe acute respiratory syndrome; SPM, Spontaneous pneumomediastinum; H1N1, Swine-Origin Influenza A
Abstract
Spontaneous pneumomediastinum (SPM) and Loculated pneumothorax (LPNX) are both generally rare clinical and radiological conditions associated with Coronavirus Disease 2019 (COVID-19). We report for the first time clinical data and radiological chest CT imaging of two patients affected by COVID-pneumonia associated with early radiological findings of SPM and LPNX.
Introduction
An increased number of cases of pneumonia with 'unknown cause' were initially reported in December 2019 in Wuhan City, Hubei Province, China. The cause of this type of pneumonia was found to be related to a newly discovered SARS-CoV-2 coronavirus responsible for the Coronavirus Disease 2019 (COVID-19). Despite travel restrictions, border controls, and quarantine measures applied all over the world, the disease was declared a pandemic on March 11, 2020. SARS-CoV-2 belongs to the β-coronavirus family, which also includes the virus responsible for severe acute respiratory syndromes (SARS) and Middle East respiratory syndromes (MERS) (SARS-CoV and MERS-CoV, respectively). SARS-CoV-2 appears to share with SARS-CoV the same human cell receptor, the angiotensin-converting enzyme 2 (ACE2), while MERS-CoV uses dipeptidyl peptidase 4 (DPP4) to enter host cells.1 , 2
Although the diagnosis of COVID-19 is currently carried out using real-time reverse transcriptase polymerase chain reaction (RT-PCR), chest computed tomography (CT) plays a key role in the early diagnosis of SARS-CoV-2 pneumonia and is recommended for assessing the extent of the disease and for monitoring pneumonia evolution during follow-up.3 , 4
Spontaneous pneumomediastinum (SPM) is a rare clinical condition defined as the presence of free air in the mediastinal structures without an apparent cause, such as trauma. SPM occurs predominantly in young males.5 There are some predisposing and precipitating factors such as asthma, respiratory infections, inhaled drug use, corticosteroids, inhalation of irritants, and other conditions as well as some anatomical predisposing alterations including tracheomalacia.5, 6, 7, 8 SPM is rarely reported in patients affected by COVID-19 pneumonia, and it could represent a potential radiological indicator of progression.9
Loculated pneumothorax (LPNX) is also a rare condition and is mainly associated with acute distress respiratory syndrome (ARDS) in patients who are mechanically ventilated.10 , 11 To the best of our knowledge, typical radiological findings of LPNX have not yet been reported in COVID-19 infection.
This paper reports the clinical data of two patients affected by COVID-19 that presented with two unusual radiological reports on a CT scan, including SPM and LPNX.
Case 1
A 78-year-old Italian woman with a previous history of diabetes mellitus and hypertension was admitted to the emergency room due to the presence of cough, fever, dyspnoea, and chest pain. The patient was reported to have been in close contact with a COVID-19 patient in a nursing home. She showed fever (38.5°C), cardiac palpitations with inspiratory and expiratory crackles on chest examination and diffuse reduced vesicular breathing. Blood tests revealed a mild leukopenia (3.6 × 10^3/μL), elevated lactate dehydrogenase (316 mg/L), and elevated C-reactive protein (27.4mg/L). The other laboratory values, including renal and hepatic laboratory examinations, were within the normal range. Arterial blood gas revealed respiratory failure with a SO2 of 86%. RT-PCR analysis of sputum samples resulted positive for SARS-CoV-2.
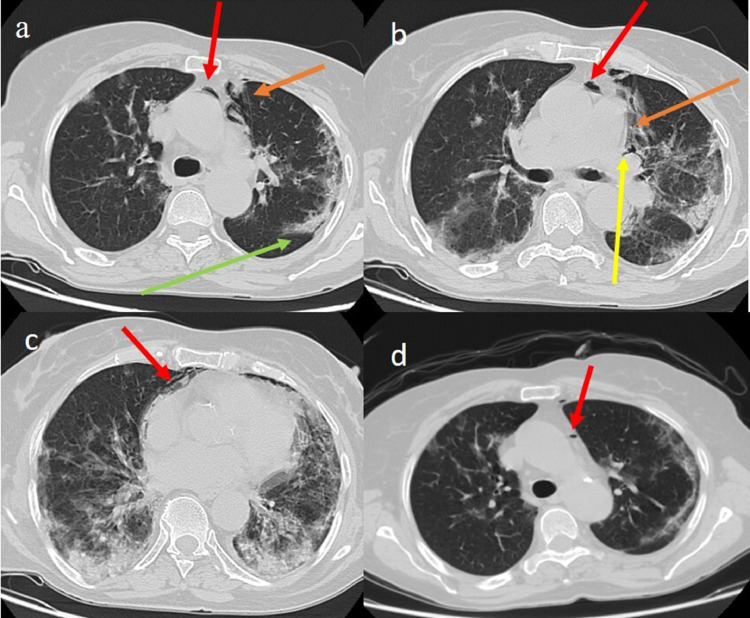
The patient's chest CT scan showed a classic appearance of COVID-19 pneumonia with septal thickness and consolidation areas in a peripheral distribution in the superior lobes, and more extensive consolidation areas in the inferior lobes (Fig. 1 a, c). The CT scan also revealed the presence of pneumomediastinum in the anterior compartment with air collection along the left perihilar area, the perivascular connective tissue and in the peripheral area related to the Meckline effect (Fig. 1 a, b, c).
Fig. 1.
Chest CT COVID-19 pneumonia with septal thickness and consolidation areas in the superior lobes (green arrow) with SPM in the anterior compartment (red arrow) and some peripheral air bubbles along the lung periphery (orange arrow) (a); air collections along the left perihilar area (yellow arrow) and the perivascular connective tissue (orange arrow); pneumopericardium was also seen (red arrow) and lung consolidations in the inferior lobes (c); reduction of the SPM (red arrow) on the CT control performed after 1 week (d). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Treatment with antiviral therapy (lopinavir/ritonavir 200/50mg twice daily orally) was thus started together with antibiotic therapy (azithromycin 2g once daily, intravenously), corticosteroid (methylprednisolone, 8mg twice daily orally) and low molecular weight heparin (2000UI). Additionally, oxygen therapy and intermittent non-invasive respiratory support (NIV) were administered. The CT scan performed one week later showed a pneumomediastinum reduction (Fig. 1 d).
Case 2
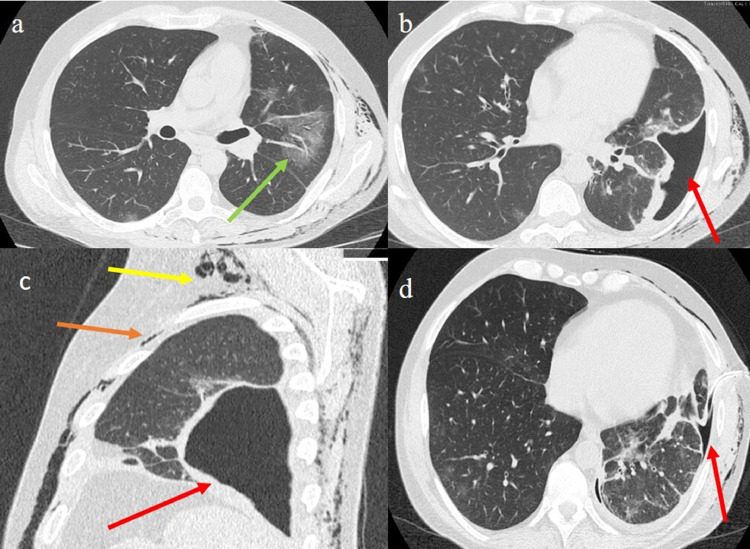
A 41-year-old Italian man was admitted to the emergency room due to a high fever (39°C), myalgia, dyspnoea, and chest pain. The patient had not reported any previous lung pathology or history of smoking. A subcutaneous emphysema over both chest walls and the neck was found during physical examination. Blood tests revealed a mild leukopenia (4.6 × 10^3/mL), low platelet count (170.000/mm3), with mildly elevated lactate dehydrogenase (190 mg/L) and D-dimer value (334ng/mL). The other laboratory values were within the normal range. Arterial blood gas revealed respiratory failure with an SO2 of 82%. RT-PCR analysis of sputum samples resulted positive for SARS-CoV-2. A chest CT scan revealed ground-glass opacities in the left superior lobe with peripheral and posterior distributions (Fig. 2 a), and some consolidation in the left inferior lobe. A LPNX was also found in the left inferior lobe (Fig. 2b), associated with pneumomediastinum and subcutaneous emphysema (Fig. 2c). In addition, a bronchoscopy revealed no bronchopleural fistula.
Fig. 2.
Chest CT COVID-19 pneumonia with ground-glass opacities (green arrow) in the left superior lobe on axial plane (a); LPNX in the left inferior lobe (red arrow) on axial plane; LPNX (red arrow) on sagittal plane, pneumomediastinum (orange arrow) and subcutaneous emphysema (yellow arrow) were also seen (c); reduction of the LPNX (red arrow) after intercostal chest drain on Chest CT control performed 5 days later (d). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
Treatment was started with hydroxychloroquine sulfate (200 mg twice daily, orally) in association with lopinavir/ritonavir (200/50 mg twice daily orally), and low molecular weight heparin (2000 UI). Corticosteroid therapy (methylprednisolone, 8 mg twice day orally in the initial dose and afterwards, 4 mg daily, orally as maintenance therapy) was also administered in association with tocilizumab (8 mg in two administrations in intravenous infusion), and oxygen therapy. The loculated pneumothorax was treated with an intercostal chest drain and the clinical condition. The chest CT scan control of the patient five days later showed an improvement (Fig. 2d).
Discussion
Imaging with chest CT plays a key role in determining the change in chest findings associated with COVID-19 pneumonia from initial diagnosis until patient recovery. The most common imaging features on a CT typical of COVID-19 infection include bilateral, multilobar ground glass opacities (GGO) with a peripheral or posterior distribution (or both), above all in the lower lobes and less frequently within the right middle lobe.3 CT follow-up studies revealed that in patients affected by COVID-19, the number and size of GGOs progressively increased, changing into multifocal consolidation areas.3 , 4 SPM has seldom been described in COVID-19.
SPM is a rare and generally benign condition defined as the presence of air in the mediastinum in the absence of a traumatic event or an iatrogenic cause as endotracheal intubation. SPM can be a diagnostic challenge given that its clinical presentation is similar to many respiratory pathologies. This clinical condition can be caused by a leakage due to wall ruptures of marginal pulmonal alveoli, secondary to high interalveolar pressure caused by factors such as artificial ventilation, coughing or straining. SPM usually originates from air migration from ruptured alveoli to the mediastinum through the Macklin effect.5, 6, 7
In the presence of a pressure gradient between an alveolus and the interstitium, the air ruptures from the alveolus into the perivascular and peribronchial fascial sheath toward the mediastinum, which can extend to the cervical subcutaneous tissue, pleura, pericardium, peritoneal cavity, and epidural space.5, 6, 7 However, in some cases, air leakage can also have an abdominal origin.12
SPM can lead to other complications such as pneumothorax (PNX) and extensive subcutaneous emphysema7 , 13; in addition SPM can cause an uncommon complication of lung infections such as staphylococcal pneumonia and fungal pneumonia.14 A few cases of SPM have also been reported in Swine-Origin Influenza A (H1N1) and in SARS infections.13 , 14 It has also been reported by Peiris,15 who showed the occurrence of SPM in 12% of SARS patients not related to intubation and mechanical ventilation. Zhou et al9 described one case of SPM in a young man affected by COVID-19, who developed COVID-19 some days later, suggesting a progressive evolution of pneumonia. A few other cases reported SPM associated with PNX and subcutaneous emphysema in patients with COVID-19.16 , 17 Wang et al16 described the case of an SPM with PNX developed during the course of COVID-19 pneumonia and with a spontaneous resolution. In the case reported by Sun et al17 the spontaneous PNX was sustained by the rupture of a giant bulla formed during the disease's progression as the result of lung parenchymal destruction.
Similar findings of SPM with PNX have been reported in patients with H1N1 infection and in those with SARS.18 , 19 Spontaneous PNX has been found as a complication in 1.7% of SARS patients.19 The histological findings in patients who died from SARS support the hypothesis of severe pulmonary injury predisposing the patient to spontaneous PNX.19 It has been suggested that a dysregulation of the immune response related to SARS-CoV-2, SARS-coV or MERS-CoV infection could lead to lung injury and the clinical and radiological findings typical of ARDS.2 , 20
Most of the cases of SPM and PNX described in patients with COVID-19 pneumonia and in those affected by SARS have some features in common, including the absence of smoking history.16 , 19 Aggressive steroid therapy has also been speculated to play a role in the pathogenesis of spontaneous PNX in SARS patients due to the fact that steroids may delay wound healing and perpetuate air leakage.19 However, other studies have not confirmed this theory because steroids are useful in controlling the rapid and damaging host inflammatory response that is usually seen in viral pneumonia.19 , 21 , 22 More frequently, SPM associated with PNX in patients with COVID-19 have been complications of tracheal intubation or mechanical ventilation in patients with chronic obstructive pulmonary disease that needed invasive ventilation for correcting hypoxemia.23 , 24
Chest CT scans are the best way to identify SPM and its complications as well as to find the Macklin effect which is usually evident as linear collections of air contiguous to the bronchovascular sheaths.5
On the other hand, LPNX is a rare form of localized pneumothorax mainly associated with ARDS, pleural malignancy, and pleural infection such as pleural aspergillosis.11 , 25 , 26 It can also be caused by the adherence of an inflamed pleura to the chest wall, which may confine a pneumothorax to a focal portion of the pleural space around the site of the air leak.26 A CT scan is a useful tool to evaluate LPNX and to distinguish it from emphysematous bulla.26 To date, only few cases of LPNX related to SARS have been reported. Sihoe et al19 described a case of a 47-year-old male patient affected by SARS complicated by a LPNX, similarly to our case.
In this paper we have reported two rare radiological findings in two patients affected by COVID-19 characterized by a sudden onset of SPM and LPNX. Both SPM and LPNX found in our cases could be related to a direct action linked to viral inflammation in the pulmonary alveolar epithelium or to an indirect viral action through persistent coughing or straining.
Conclusions
We have described the use of chest CT scans that revealed the presence of both SPM and LPNX in two patients affected by COVID -19, as an expression of a rapid and aggressive action of the viral spread in immunological and genetically predisposed individuals. These radiological findings highlight the importance of using early chest CT scans in order to identify more detailed radiological findings and to start a more specific treatment.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the patients.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors have no conflict of interests to disclose. Informed consent was obtained from the patients. We declare that this study has been read and approved by all authors and that all authors agree to the submission of the manuscript to the Journal. All named authors had an active contribution to the conception and design and/or analysis and interpretation of the data and/or the drafting of the paper and all had critically reviewed its content and have approved the final version submitted for publication. We declare that the study has not been published previously, that it is not under consideration for publication elsewhere. The research did not receive any specific grant from funding agencies in the public, commercial, or profit sectors.
References
- 1.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. 2020. [DOI] [PubMed] [Google Scholar]
- 3.Dong J., Peng H., Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. AJR. 2020:1–6. doi: 10.2214/AJR.20.22961. [DOI] [PubMed] [Google Scholar]
- 4.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murayama S., Gibo S. Spontaneous pneumomediastinum and Macklin effect: overview and appearance on computed tomography. World J Radiol. 2014;6(11):850. doi: 10.4329/wjr.v6.i11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zylak C.M., Standen J.R., Barnes G.R., Zylak C.J. Pneumomediastinum revisited. Radiographics. 2000;20(4):1043–1057. doi: 10.1148/radiographics.20.4.g00jl131043. [DOI] [PubMed] [Google Scholar]
- 7.Meireles J., Neves S., Castro A., França M. Spontaneous pneumomediastinum revisited. Respir Med CME. 2011;4(4):181–183. doi: 10.1016/j.rmedc.2011.03.00. [DOI] [Google Scholar]
- 8.Lal A., Mishra A.K., Sahu K.K., Noreldin M. Spontaneous pneumomediastinum: rare complication of tracheomalacia. Arch bronconeumol. 2019;56(3):185–186. doi: 10.1016/j.arbres.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C., Gao C., Xie Y., Xu M. COVID-19 with spontaneous pneumomediastinum. Lancet Infect Dis. 2020;20(4):510. doi: 10.1016/S1473-3099(20)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland G.W., Lee M.J., Sutcliffe N.P., Mueller P.R. Loculated pneumothoraces in patients with acute respiratory disease treated with mechanical ventilation: preliminary observations after image-guided drainage. J Vasc Interv Radiol. 1996;7(2):247–252. doi: 10.1016/s1051-0443(96)70771-6. [DOI] [PubMed] [Google Scholar]
- 11.Isaac B.T.J., Samuel J.T., Mukherjee D.K., Pittman M. Loculated pneumothorax due to a rare combination resulting in an interesting chest radiograph. Clin Respir J. 2017;11(6):1074–1078. doi: 10.1111/crj.12457. [DOI] [PubMed] [Google Scholar]
- 12.Sahu K.K., Sherif A.A., Mishra A.K., Vyas S., George S.V. Perineal ulcer: a rare cause of extensive subcutaneous emphysema. BMJ Case Rep. 2019;12(4) doi: 10.1136/bcr-2019-229918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chekkoth S.M., Supreeth R.N., Valsala N., Kumar P., Raja R.S. Spontaneous pneumomediastinum in H1N1 infection: uncommon complication of a common infection. J R Coll of Physicians Edinb. 2019;49(4):298. doi: 10.4997/JRCPE.2019.409. [DOI] [PubMed] [Google Scholar]
- 14.Chu C.M., Leung Y.Y., Hui J.Y.H. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23(6):802–804. doi: 10.1183/09031936.04.00096404. [DOI] [PubMed] [Google Scholar]
- 15.Peiris J.S.M., Chu C.M., Cheng V.C.C. Clinical progression and viral load in a community outbreak of coronavirus associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W., Gao R., Zheng Y., Jiang L. COVID-19 with spontaneous pneumothorax, pneumomediastinum and subcutaneous emphysema. J Travel Med. 2020 doi: 10.1093/jtm/taaa062. taaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun R., Liu H., Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21(5):541. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bor C., Demirağ K., Uyar M., Çankayalı İ., Moral A.R. Recurrent spontaneous pneumothorax during the recovery phase of ARDS Due to H1N1 Infection. Balkan Med J. 2013;30(1):123. doi: 10.5152/balkanmedj.2012.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sihoe A.D., Wong R.H., Lee Severe acute respiratory syndrome complicated by spontaneous pneumothorax. Chest. 2004;125(6):2345–2351. doi: 10.1378/chest.125.6.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brogna B., Brogna C., Musto L. SARS-CoV-2 infection with different radiological insights. Diagnostics. 2020;10(5):283. doi: 10.3390/diagnostics10050283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson B., Cockram C. SARS: experience at Prince of Wales Hospital, Hong Kong. Lancet. 2003;361:1486–1487. doi: 10.1016/S0140-6736(03)13218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui D.S.C., Sung J.J.Y. Severe acute respiratory syndrome. Chest. 2003;124:12–15. doi: 10.1378/chest.124.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radiopedia. Post-intubation pneumomediastinum and pneumothorax - background COVID-19 pneumonia. https://radiopaedia.org/cases/post-intubation-pneumomediastinum-and-pneumothorax-background-covid-19-pneumonia?lang=us
- 24.Xiang C., Wu G. SARS-CoV-2 pneumonia with subcutaneous emphysema, mediastinal emphysema, and pneumothorax: A case report. Medicine (Baltimore) 2020;99(20):e20208. doi: 10.1097/MD.0000000000020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirzai S, Vashisht R, Ghosh S. Loculated pneumothorax masquerading as bullous disease. Am J Med Sci. 2020 doi: 10.1016/j.amjms.2020.04.008. 2020S0002-9629(20)30125-7. [DOI] [PubMed] [Google Scholar]
- 26.O'connor A.R., Morgan W.E. Radiological review of pneumothorax. BMJ. 2005;330(7506):1493–1497. doi: 10.1136/bmj.330.7506.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]