Abstract
The practice of social distancing and wearing masks has been popular worldwide in combating the contraction of COVID-19. Undeniably, although such practices help control the COVID-19 pandemic to a greater extent, the complete control of virus-laden droplet and aerosol transmission by such practices is poorly understood. This review paper intends to outline the literature concerning the transmission of virus-laden droplets and aerosols in different environmental settings and demonstrates the behavior of droplets and aerosols resulted from a cough-jet of an infected person in various confined spaces. The case studies that have come out in different countries have, with prima facie evidence, manifested that the airborne transmission plays a profound role in contracting susceptible hosts. The infection propensities in confined spaces (airplane, passenger car, and healthcare center) by the transmission of droplets and aerosols under varying ventilation conditions were discussed.
Interestingly, the nosocomial transmission by airborne SARS-CoV-2 virus-laden aerosols in healthcare facilities may be plausible. Hence, clearly defined, science-based administrative, clinical, and physical measures are of paramount importance to eradicate the COVID-19 pandemic from the world.
Keywords: Airborne transmission, Coronavirus, Lockdown, Masks, SARS-CoV-2
Highlights
-
•
Social distancing and wearing masks became popular worldwide for control of COVID-19.
-
•
Droplet and aerosol transmission of COVID-19 has been poorly understood.
-
•
Examples have been reported to speculate the airborne transmission of COVID-19.
-
•
Nosocomial infection through airborne transmission seems plausible in confined spaces.
-
•
Proper administrative, clinical, and physical measures are paramount to combat the transmission.
1. Introduction
Coronavirus disease 2019 (COVID-19) was first reported in Wuhan, China, in December 2019 (Chen et al., 2020). The disease is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) (Gorbalenya, 2020) and asseverated to be transmitted from human-to-human by multiple means, namely, by droplets, aerosols, and fomites (Wang and Du, 2020). It has been more than 120 days that COVID-19, later declared as a pandemic and highly contagious, was first reported. As of May 05, 2020, there have been more than 3.5 million confirmed cases and 243,401 deaths by the COVID-19 disease worldwide (WHO, 2020a). COVID-19 infection triggers severe acute respiratory illness, with fever, cough, myalgia, and fatigue as common symptoms at the onset of illness (Huang et al., 2020; Judson and Munster, 2019; Nicas et al., 2005).
Infectious agents may spread from their natural reservoir to a susceptible host in different pathways. There are various classifications reported in the literature for modes of transmission of different infectious agents. Morawska (2006) has presented a classification for virus transmission, including human-human transmission, airborne transmission, and other means of transmission such as endogenous infection, common vehicle, and vector spread. However, many respiratory viruses are believed to transmit over multiple routes, of which droplet and aerosol transmission paths become paramount, but their significance in transmitting the disease remains unclear (Morawska and Cao, 2020; Shiu et al., 2019). In general, infected people spread viral particles whenever they talk, breathe, cough, or sneeze. Such viral particles are known to be encapsulated in globs of mucus, saliva, and water, and the fate/behavior of globs in the environment depends on the size of the globs. Bigger globs fall faster than they evaporate so that they splash down nearby in the form of droplets (Grayson et al., 2016; Liu et al., 2016). Smaller globs evaporate faster in the form of aerosols, and linger in the air, and drift farther away than the droplets do.
Respiratory particles may often be distinguished to be droplets or aerosols based on the particle size and specifically in terms of the aerodynamic diameter (Hinds, 1999). One could dispute that, unlike larger droplets, aerosols may pose a greater risk of the spread of the COVID-19 disease among many susceptible hosts positioned far from the point of origin. Nevertheless, it has been proven that viral disease outbreaks via aerosol transmission are not as severe as one would think, because of dilution and inactivation of viruses that linger for extended periods in the air (Shiu et al., 2019). There has been no discernible evidence on the minimum infectious viral load for COVID-19 pandemic, but many researchers speculate that a few hundreds of SARS-CoV-2 virus would be enough to cause the disease among susceptible hosts (Beggs, 2020; SMC, 2020).
There have been numerous disagreements on the average particle size of droplets and aerosols (Shiu et al., 2019). The World Health Organization (WHO) and Centers for Disease Control and Prevention (CDC) postulate that the particles of more than 5 μm as droplets, and those less than 5 μm as aerosols or droplet nuclei (Siegel et al., 2007; WHO, 2014). Conversely, there have been some other postulations, indicating that aerodynamic diameter of 20 μm or 10 μm or less should be reckoned to be aerosols, based on their ability to linger in the air for a prolonged period, and the reachability to the respirable fraction of the lung (alveolar region) (Gralton et al., 2011; Nicas et al., 2005; Tellier, 2009). Small aerosols are more susceptible to be inhaled deep into the lung, which causes infection in the alveolar tissues of the lower respiratory tract, while large droplets are trapped in the upper airways (Thomas, 2013). For easy apprehension, aerosols can be defined as suspensions of solid or liquid particles in the air, which can be generated by either natural or anthropogenic phenomena (Judson and Munster, 2019; Tellier, 2009).
Though social distancing would be promising in combatting the COVID-19, the minimum distances that have been maintained between an infected person and a host are disputable and far from being established based on any scientific evidence. Nevertheless, many have believed that droplets predominate over aerosols in terms of contracting the disease; thus, over time, research work has been focused on acquiring better knowledge on the science of droplet transmission (Morawska and Cao, 2020; Wang and Du, 2020). However, since the recent past, evidence has been provided to refute the former hypothesis and speculated that aerosols also play a major role in transmitting the disease (Morawska and Cao, 2020; Wang and Du, 2020). As such, the controversy on the modes of transmission of the SARS-CoV-2 virus seems to be speculating and puzzled among many researchers, including the WHO (Morawska and Cao, 2020). No conclusive studies have been conducted on differentiating between the modes of transmission of viruses via droplets and aerosols; hence, unresolved dichotomy.
It has also been argued that environmental settings, in which the SARS-CoV-2 virus transmits, trigger the disease adversely or beneficially with a susceptible host exposed to more or lesser payloads, respectively (Morawska, 2006; Tellier et al., 2019). Such adverse or beneficial scenarios are based on plausible changes in the fate of the virus in the environment caused by altered transport phenomena. There have been myriads of hypotheses corroborating that certain threshold levels of humidity, temperature, sunlight, and ventilation will speed up the virus-laden droplet and aerosol transmission, aggravating the spread of the SARS-CoV disease (Morawska, 2006).
As scientists underpin more conclusive evidence on the modes of transmission via droplets and aerosols, facemasks and respirators worn by billions of people around the globe (both infected persons and susceptible hosts) become a common sight in day-to-day activities. In the events of the droplet and aerosol transmission, the efficacy of such personal protective equipment in combating the transmission of the SARS-CoV-2 has been poorly understood.
Ever since the COVID-19 has been declared to be a pandemic with incredibly high morbidities and mortalities worldwide, the database of research on controlling the COVID-19, especially in the indoor environment, has been updated with several evidence-based studies. However, less attention has been focused on the whole in controlling virus-laden droplet and aerosol shedding, their transport phenomena, and plausible methods of their dilution and destruction in different indoor settings. With more COVID-19 cases reported worldwide, evidence-based decisions need to be adhered to in combating the disease, especially for situations in confined environments. The transmission of droplets and aerosols within confined spaces becomes profoundly complex phenomena, and the real trajectories under different micro-climatic conditions are poorly understood. The aggressive nature of the disease is directly connected with the transport phenomena of both droplets and aerosols, and the comprehension of such phenomena is vital in controlling the spread of the disease within such confined spaces. Aerodynamic engineers, therefore, need to network with virologists to fully understand the possible trajectories of the viral spread within such confined spaces. In this context, computational fluid dynamics could be made use of, to simulate the trajectories resulting from coughs and sneezes of an infected person within different confined settings.
This review paper is divided into two parts: Part 1 underpins the basic principles underlying the transmission via droplets and aerosols (Sections 2–5), and Part 2, being the common practices adopted by many in controlling the COVID-19 transmission with different masks worn in three confined settings; airplane, passenger car, and healthcare center (Section 6). Fig. 1 depicts an explicative schema of the two parts described in the following sections.
Fig. 1.
Part-1 enumerates the principles and findings on the transmission of virus-laden droplets and aerosols in literature, and Part-2 deliberates practices that are common in confined settings under different ventilation scenarios.
Part 1:
2. Sources and mechanisms of generating and transmitting droplets and aerosols
Although the direct transmission from infected person/s is the primary source of aerosols and droplets, other scenarios such as medical procedures, surgeries (Judson and Munster, 2019), fast-running tap water and toilet flushes (Morawska, 2006) also generate aerosols contaminated with infectious pathogens. The most common types of viruses causing infections in the respiratory tract through aerosol transmission are influenza viruses, rhinoviruses, coronaviruses, respiratory syncytial viruses (RSVs), and parainfluenza viruses (Morawska, 2006). Tellier (2009) has postulated three modes in which the influenza virus can be transmitted: aerosol transmission, droplet transmission, and self-inoculation of the nasal mucosa by contaminated hands. Another classification is presented by Judson and Munster (2019), which is often referred to as the term of ‘airborne transmission’ to describe the disease spread by small droplet aerosols and droplet nuclei, while the term ‘droplet transmission’ to describe infection by large droplet aerosols. The term ‘airborne transmission’ defined by Morawska (2006) is quite similar to the same apprehended by Judson and Munster (2019). Besides, the direct contact and fomite transmission produced by aerosol-generating medical procedures (AGMPs) can also be considered as potential transmission pathways (Judson and Munster, 2019).
Droplet transmission occurs by the direct spray of large droplets onto conjunctiva or mucous membranes of a susceptible host when an infected patient sneezes, talks, or coughs. In the meantime, direct physical touch between an infected individual and susceptible host and indirect contact with infectious secretions on fomites can cause the contact transmission (Boone and Gerba, 2007; Brankston et al., 2007; Nicas et al., 2005; Tellier, 2006).
It is a well-known fact that COVID-19 is transmitted by human-to-human contact; hence, contagious. One of the predominant mechanisms for COVID-19 to be contagious is self-inoculation from contaminated fomites. Self-inoculation could occur by poor hand hygiene (Kwok et al., 2015) or by not following the common disease-controlling etiquettes. The viral transmission because of the frequent touches of contaminated fomites was found to be a source of the disease. Consequently, many researchers have paid attention to the airborne transmission directly by virus-laden droplets and aerosols. However, the novelty of this viral outbreak limits the prima facie evidence to determine the potential transmission routes, and thus, it is assumed that SARS-CoV-2 also spreads as the other human coronaviruses (CDC, 2020a).
Recent studies corroborated that COVID-19 is transmitted primarily between people through respiratory droplets and contact routes (Burke, 2020; CDC, 2020a; Chan et al., 2020; Huang et al., 2020; Li et al., 2020; Liu et al., 2020b; WHO, 2020b). Besides, evidence has been found that fecal contamination caused by an infected person is discernible to spread the SARS-CoV-2 virus (Zhang et al., 2020). A recent study in China has investigated 1,070 specimens collected from 205 infected patients at three hospitals in the Hubei and Shandong Provinces, and about 29% of positive cases for COVID-19 have been observed with the transmission through feces (Wang et al., 2020c). Further, they also highlighted the fact that COVID-19 could be transmitted via fecal routes after they detected the live infectious agents of COVID-19 in patients' stools (Wang et al., 2020c). Contrary to what has been stated above, the WHO, at early hours of manifestation of COVID-19, has denounced that there was no supporting evidence on the fecal-oral transmission of the SARS-CoV-2 virus (WHO, 2020b). The same report also highlighted the fact that airborne transmission has not played a significant role in disease transmission from 75,465 confirmed COVID-19 cases in China as of March 27, 2020 (WHO, 2020c). In contrast to the WHO study, another study has reported that SARS-CoV-2 can survive in the air for many hours, causing potential aerosolized transmission (van Doremalen et al., 2020). With more infected persons being recorded in many countries, the WHO has intimated that certain hospital procedures would also generate aerosols under specific circumstances: endotracheal intubation, bronchoscopy, open suctioning, administration of nebulized treatment, manual ventilation before intubation, turning the patient to the prone position, disconnecting the patient from the ventilator, non-invasive positive-pressure ventilation, tracheostomy, and cardiopulmonary resuscitation (WHO, 2020b). As precautions to prevent such plausible airborne transmission of viruses, the WHO has recommended a myriad of management protocols (WHO, 2020d).
Besides, healthcare workers are unwittingly exposed to infectious agents through person-to-person contact via respiratory droplets or aerosols and direct handling of contagious secretions (e.g., sputum, serum, blood, feces, etc.) of COVID-19 patients. Ong et al. (2020) have studied the sources of COVID-19 that could transmit the infection during the involvement in healthcare services. The results obtained from their study indicate that the samples collected from the personal protective equipment (PPE) worn by the hospital staff (physicians exiting the patient rooms) were negative for COVID-19. However, the samples from the air outlet exhaust fans in patient-rooms except corridors and anterooms have been reported as positive for COVID-19, indicating that the airborne transmission is plausible. Sean et al. (2020) corroborated that swabs taken from air exhaust outlets in a hospital room of a symptomatic patient of COVID-19 in Singapore tested positive, suggesting that small virus-laden aerosols have been displaced by airflows and deposited on equipment such as vents. However, there is no conclusive evidence as to how it is contaminated, and it is presumed that the aerosol particles may have got deposited in the vent. On February 3, 2020, in Inner Mongolia of China, there has been a case of COVID-19 reported positive when a person has passed the door of a symptomatic patient several times, giving evidence of the airborne transmission (Wang and Du, 2020).
3. Size distribution, time taken, and distances transmitted by aerosols and droplets produced by infected people
The SARS-CoV-2 is often said to be transmitted through droplets generated when a symptomatic person coughs, sneezes, talks, or exhales (Morawska and Cao, 2020). Some of these droplets are too heavy to remain in the air, and rather fall on nearby floors or surfaces. Fomites collect droplets contaminated with SARS-CoV-2, and touching of such surfaces by a susceptible host would get infected. However, some droplets, when ejected from an infected person, convert to aerosol particles (also known as bioaerosols) with relatively smaller aerodynamic diameters and, consequently, become airborne (Morawska, 2006). Such virus-laden aerosol particles are capable of infecting people who inhale such particles, thereby spreading the disease. Further, there have been several transport phenomena where larger droplets become smaller through evaporation so that such smaller particles are called droplet nuclei. Such aerosol particles with the encapsulation of viruses could be termed as bioaerosols or droplet nuclei; hence, the term ‘aerosol’, ‘bioaerosol’, and ‘droplet nuclei’ is used in this paper interchangeably. The scenarios in respect of the generation of droplets and aerosol, particularly in the indoor environment, have not been adequately understood, and thus, insights into the plausible mechanisms are worthy of being explored. Duguid (1945), for the first time, has explored the characteristics of droplets and aerosol from human expiratory activities with chest infections, and such information is presented in Table 1 . Duguid (1945) has observed that 95% of particles were often smaller than 100 μm, and the majority were between 4 and 8 μm. The findings corroborated that breathing and exhalation originated from the nose have shed up to a few hundreds of droplets of which some were aerosols. In contrast, talking, coughing, and sneezing have produced more aerosols than droplets (Table 1).
Table 1.
Detailed information of droplets and aerosols generated from human expiratory activities (Source: Duguid, 1945).
| Activity | Number of droplets and aerosols generated (1–100 μm) | Presence of aerosols (1–2 μm) | Region of origin |
|---|---|---|---|
| Normal breathing (for 5 min) | None – few | Some | Nose |
| Single strong nasal expiration | Few – few hundred | Some | Nose |
| Counting loudly - talking | Few dozen – few hundred | Mostly | Front of the mouth |
| A single cough (mouth open) | None – few hundred | Some | Faucial region |
| A single cough (mouth initially closed) | Few hundred – many thousand | Mostly | Front of the mouth |
| Single sneeze | Few hundred thousand – few million | Mostly | Front of the mouth |
| Few – few thousand | Some | Both from the nose and the faucial region |
On the contrary to what Duguid (1945) has presented, a study conducted by Papineni and Rosenthal (1997) with five healthy individuals has manifested that 80–90% of particles from human expiratory activities were aerosols with the diameter being smaller than 1 μm. The study also corroborated that the highest aerosol densities were generated during coughing and the lowest from nasal breathing, of which exhaled breath would be more responsible in transmitting the viruses (size of the order of 0.1 μm) when compared with transmitting the bacteria (> 1 μm). It has been found that vomiting by a SARS-CoV infected person in the corridor of a hotel in Hong Kong in 2003 has contracted the disease on several people nearby by aerosol transmission (Morawska, 2006).
The physicochemical processes affecting the fate of airborne aerosols constitute evaporation, interaction with other types of particles, transport, and removal from the air by deposition on solid surfaces (Morawska, 2006). Particles in the air are often subjected to Brownian motion, gravity, electrostatic forces, thermal gradients, electromagnetic radiation, turbulent diffusion, and inertial forces (Baron and Willeke, 2001). Of these mechanisms, the diffusion is a key mechanism of transmitting viruses with particles in the lower sub-micrometer range, together with other aerosol particles (Baron and Willeke, 2001). For droplets larger than 1 μm, gravity becomes significant than Brownian motion in deciding the fate of such particles (Cox, 1995). Under the standard atmospheric conditions, droplets smaller than 100 μm often evaporate before reaching the ground, and the evaporated droplet residues linger in the air for prolonged periods (Morawska, 2006). When the droplets contain infectious bioaerosols, such as viruses, bioaerosols will remain in the air, even after the liquid content evaporates (Morawska, 2006). However, the time interval that a virus survives in the air varies from one type of bioaerosol to another type. Droplets in the range of 0.5–20.0 μm lingering in the air are more likely to be retained in the respiratory tract and produce the infection (McCluskey et al., 1996). However, droplets seem to be not present in the air for longer periods; instead, evaporation takes place, transforming droplets to bioaerosol residues, which could linger in the air for extended periods.
Hui and Chan (2010) have investigated that in different indoor environments, SARS-CoV could be transmitted through the airborne route. Another retrospective study has found that the airborne transmission in an aircraft from an infected person to passengers located seven rows of seats ahead, indicating that the SARS-CoV virus could travel for a distance more than 1 m horizontally (Olsen et al., 2003). Another case has been reported on infecting more than 1,000 persons in an apartment complex in Hong Kong because of aerosols generated by the building's sewage system (McKinney et al., 2006). These observations manifest that the aerosol-laden SARS-CoV virus transmission is a phenomenon, which would impart greater havoc than one thinks, and precautionary measures are, therefore, of paramount importance.
The SARS-CoV-2 virus has been found to remain viable in aerosols for 3 h, while it, in the form of droplets, is more stable on plastic and stainless steel, copper, cardboard, and glass with durations detected up to 72, 4, 24, and 84 h, respectively (van Doremalen et al., 2020). In comparison, the SARS-CoV virus was also found to be airborne in the form of aerosols for 3 h, indicating that both SARS viruses behave more or less in the same manner in the air. Nevertheless, the SARS-CoV virus remains stable and viable in the form of droplets on plastic and stainless steel, copper, cardboard, and glass with durations (half-lives) lasting to 72, 8, 8, and 96 h, respectively (van Doremalen et al., 2020). The half-lives of the SARS-CoV-2 and SARS-CoV are almost the same in aerosols, with median estimates of approximately 1.1–1.2 h, indicating that both viruses have similar stability characteristics in transmitting through the air (van Doremalen et al., 2020). However, more profound epidemiological sustenance of SARS-CoV-2 virus may, therefore, be because of some other factors, including high viral loads in the upper respiratory tract and the capability of persons infected with COVID-19 to shed and transmit the virus while remaining asymptomatic (Bai et al., 2020; Zou et al., 2020).
Based on a study carried out by Nicas et al. (2005), it has been estimated that particles emitted from a cough of an infected person of a respiratory illness quickly decrease in diameter (with initial diameters of less than 20 μm) mainly because of the water loss by approximately half of the initial volume, amounting to 6 × 10−8 mL. Exhaust ventilation, particle settling, die-off, and air disinfection methods are some prominent mechanisms by which the removal of viable airborne pathogens often takes place; each removal mechanism follows a first-order reduction rate (Nicas et al., 2005). Based on 3-h viability of SARS-CoV-2 in the air (van Doremalen et al., 2020), prerequisites for the disease such as exposure, inhalation, and infection could occur minutes or a few hours later near and far from an aerosol source even in a stagnant environment (Bourouiba, 2020).
The actual airborne times for droplets may be greater in an environment where there are significant cross-flows (WHO, 2009). Such scenarios could be expected in quarantine and healthcare centers (e.g., with doors opening, bed and equipment movement, and people walking back and forth, constantly). Conversely, airborne durations for smaller droplet nuclei or aerosols may be profoundly shorter when they are subject to a significant downdraft (e.g., if they pass under a ceiling supply vent) (WHO, 2009). When the flow of mucus or saliva ejects from an infected person, its trajectory is determined primarily by the size of droplets and airflow patterns that govern the paths of movement (Tang et al., 2006). The Stokes' law describes the resultant trajectory of the droplets subjected to the forces of gravity downwards and air friction upwards, which governs the droplet movement in the air (Wells, 1934). Coughs and sneezes usually constitute a turbulent cloud of buoyant gas with suspended droplets of various sizes. The larger droplets follow a ballistic trajectory irrespective of flow in the gas phase, whereas the aerosols are buoyant to a varying degree within the turbulent gas cloud (Bourouiba et al., 2014).
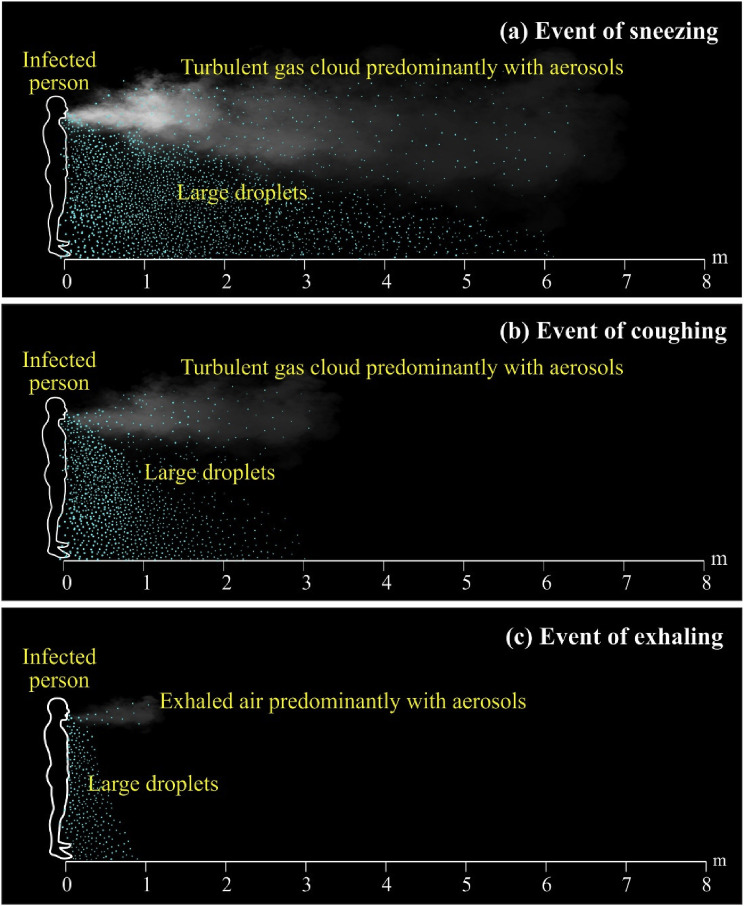
In general, there exists an accepted notion of a 2-m safe exclusion zone to prevent possible droplet transmission from an infected person to a susceptible host; however, there are no comprehensive studies to support such a phenomenon. Wells (1934) has supported the 2-m exclusion zone concept taking into account the evaporation-falling curve. Wells (1934) has postulated that large droplets (> 100 μm) will fall to the floor within a horizontal distance of 2 m from the source. Simple calculations, assumptions, and inadequate empirical data of Wells's study have been later speculated by Xie et al. (2007). Xie et al. (2007) have corroborated that for respiratory exhalation flows, the larger droplets (diameter between 60 μm and 100 μm) were, depending on the exhalation air velocity and relative humidity of the air, carried away for more than 6 m of horizontal distance with the exhaled air having a velocity of 50 m/s at the point of expiration (Fig. 2 a). Such scenarios simulate sneezing events. Conversely, larger droplets were found to carry for more than 2 m afar at a velocity of 10 m/s reordered at the point of exit, simulating coughing bouts (Fig. 2b). The same for exhaling events for which the velocity is at 1 m/s was found to carry large droplets only up to about 1 m horizontally (Fig. 2c). Other studies also have proven that when an infected person of a respiratory illness coughs or sneezes, a cloud of pathogen-bearing droplets of different sizes appears to come out and travels even up to 7–8 m from the point of source (Bourouiba et al., 2014; Bourouiba, 2016).
Fig. 2.
Trajectories of droplets and aerosols from an infected patient (a) event of sneezing with droplets travelled for 6 m at a speed of 50 m/s within 0.12 s (b) event of coughing with droplets travelled for 2 m at a speed of 10 m/s within 0.2 s (c) event of exhaling with droplets travelled for 1 m at a speed of 1 m/s within 1 s.
Moreover, recent experiments conducted after COVID-19 contagion by Bourouiba (2020) and Loh et al. (2020) have been in agreement with the findings of Xie et al. (2007). Xie et al. (2007) have reported that pathogen-bearing droplets of all sizes can travel for almost 7–8 m during sneezes and for more than 2 m (maximum of 4.5 m) during coughs. Surprisingly, there have been contradicting insights on the distance to be maintained between healthcare workers and COVID-19 infected patients [e.g., 1 m (WHO, 2020e) and 2 m (CDC, 2020b)]. However, most of the studies on the COVID-19 virus mentioned above have been carried out in laboratories with expiration devices set on manikins; hence, no convincing information can be deduced.
4. Behavior of droplets and aerosols against environmental factors
The most important environmental factors that could impact on the viability of airborne microorganisms are temperature, humidity, radiation (sunlight), and open-air (ventilation) (Marthi, 1994). Most viruses, including SARS-CoV-2, are less than 100 nm in size (Kumar and Morawska, 2019). Viruses in aerosols lose or gain the viability and infectivity because of environmental stresses caused by temperature, relative humidity, and sunlight before they reach a susceptible host. Environmental tolerance of the virus-laden aerosols depends on the specific phenotype available, the composition of the bioaerosols containing virus and their payload, and physical characteristics in the surrounding environment (Schuit et al., 2020). As the environmental factors play a major role in transmitting payloads of SARS-CoV-2 virus in different geographical locations of outdoor and indoor environments, it is worthy of exploring the effects of environmental factors on the transmission of SARS-CoV-2 virus. Furthermore, there have been associations between air pollution represented by air pollutants such as PM2.5, PM10, NO2, and O3 and COVID-19 infection (Zhu et al., 2020). SARS-CoV-2 could bind with particulate matter and could be airborne. In an indoor environment, such viral loads primarily become airborne by advective forces propelled by local ventilation patterns and travel further away through diffusion and dispersion processes. Table 2 summarizes the relationships of viral payloads resulted from different transmission routes with environmental parameters deduced by various researchers.
Table 2.
Relationships of viral payloads with environmental parameters.
| Environmental Parameter | Synthesized information | Reference |
|---|---|---|
| Daily minimum temperature with lagged effect of 5–7 days | Inverse relationship with numbers of daily SARS-CoV cases in Beijing and Hong Kong | Bi et al. (2007) |
| Air temperature at 4 °C and relative humidity (< 20% or > 80%) | Higher survival of payloads of transmissible gastroenteritis and mouse hepatitis viruses for extended days on surfaces in indoor environment | Casanova et al. (2010) |
| Temperatures of 22–25 °C and relative humidity of 40–50%, | Higher survival rates of SARS-CoV on smooth surfaces simulating typical air-conditioned environments | Chan et al. (2011) |
| Temperature at 38 °C, and relative humidity > 95% | Los of viability of SARS-CoV, simulating tropical climates | Chan et al. (2011) |
| Ambient temperature (16–28 °C) with 7-day time lag | Stimulated the growth of SARS-CoV | Tan et al. (2005) |
| Environmental temperature related to unexpected rapid spells of cold and warm days | Rise in SARS-CoV cases | Tan et al. (2005) |
| Low temperature/low humidity conditions even after 48 h (20 °C and 40% relative humidity) | More stable and viable payloads of MERS-CoV | van Doremalen et al. (2013) |
| Lower air temperatures (6 °C) and lower relative humidity (30%) than at higher relative humidity | Greater survival of coronaviruses in surfaces | Ijaz et al. (1985); Kim et al. (2007) |
| Lower air temperatures (6 °C) | Enhanced viral survival | Harper (1961) |
| Diurnal temperature | Positive relationship of daily death counts of SARS-CoV patients | Park et al. (2019) |
| Low temperatures in the absence of ultraviolet light and different relative humidity | Slowest inactivation of influenza virus | Kormuth et al. (2018); Lowen et al. (2007); McDevitt et al. (2012); Skinner and Bradish (1954); Yang et al. (2012) |
| Temperature and humidity during the winter season in temperate countries, in the rainy season, or where there were sudden seasonal changes in tropical countries | Strong association of transmission rate of the influenza virus | Biswas et al. (2014); Chowell et al. (2012); Hemmes et al. (1962); Viboud et al. (2006) |
| Absolute humidity | Negative association with daily survival counts of Influenza patients | Metz and Finn (2015) |
| Cold temperature and low relative humidity | Stimulate Influenza transmission | Lowen et al. (2007) |
| Temperature at 30 °C and at all humidity | No association with Influenza transmission | Lowen et al. (2008) |
| Absolute humidity | Wintertime increase in influenza virus transmission and influenza virus survival | Shaman and Kohn (2009) |
| Absolute humidity | No strong correlation with airborne transmission of Influenza virus | Tang et al. (2010) |
| Temperature and relative humidity | Strong correlation with airborne transmission of Influenza virus | Tang et al. (2010) |
| Sunlight | Negative relationship with survival and infectivity of various viruses | Nelson et al. (2018); Rzeżutka and Cook (2004); Tang (2009); Qiao et al. (2018) |
| Natural and simulated sunlight | Significant loss of infectivity of influenza virus in liquid suspensions and aerosols | Schuit et al. (2020); Skinner and Bradish (1954) |
| Natural and simulated sunlight | High sensitivity of SARS-CoV survival | Tseng and Li (2007); WHO (2004) |
| Natural sunlight and UV radiation | Decay the viability of SARS-CoV | Karapiperis et al. (2020) |
| 60 min of exposure to > 90 W/cm2 of UV-C light at a distance of 80 cm | Loosing viability of SARS-CoV | Duan et al. (2003) |
| 15 min of exposure to UV-C light (> 90 W/cm2) at a closer distance (< 80 cm) | High efficiency of inactivation of SARS-CoV | Darnell et al. (2004) |
| Inadequate indoor ventilation | Enhanced infection risk of SARS-CoV in makeshift hospitals | WHO (2009) |
| With > 12 air changes per hour (ACH) (e.g., equivalent to > 80 L/s for a 24 m3-room) and controlled direction of airflow | Low risk of infectivity of viral diseases in an airborne precaution room | AIA (2001); Mayhall (2004); Wenzel (2003); WHO (2007) |
| Negative pressure of > 2.5 Pa, an airflow having a difference between the exhaust to supply > 125 cfm (56 L/s), clean-to-dirty airflow, > 12 ACH for a new building, and > 6 ACH in existing buildings for an old building, and exhaust to the outside, or a HEPA-filter if room air is recirculated | Low risk of infectivity in an airborne infection isolation room | CDC (2003) |
| Ambient temperature (< 3 °C) | Positive association of daily number of SARS-CoV-2 cases | Zhu and Xie (2020) |
| Average daily ambient temperature | Significant negative correlation with SARS-CoV-2 for northern hemisphere countries | Tosepu et al. (2020) |
| Minimum temperature, maximum temperature, relative humidity, and amount of rainfall | No significant correlation with SARS-CoV-2 | Tosepu et al. (2020) |
| Increasing ambient daily average temperature up to around 13 °C | Negative association of daily number of SARS-CoV-2 cases | Oliveiros et al. (2020); Wang et al. (2020b) |
| Diurnal temperature and absolute humidity | Positive and negative associations with daily death counts of COVID-19 patients | Ma et al. (2020) |
| Poor ventilation (approximately 150 m3 per hour per person) | High infectives in makeshift hospitals in Hubei Province, China | Chen and Zhao (2020) |
| Increase of temperature and humidity | No marked relationship with SARS-CoV-2 cases in the northern hemisphere in spring and summer months | Poirier et al. (2020) |
| High temperature and high humidity | Reduced Reproductive number (R) of COVID-19 in China and USA | Wang et al. (2020a) |
| Changes in temperature | No significant correlation with SARS-CoV-2 cases transmitted, deaths or recovered | Stanam et al. (2020) |
| Temperature and humidity | Association of infectivity of SARS-CoV-2 with temperature but no association with humidity | Gupta (2020) |
| Humidity | Direct and positive correlation with COVID-19 mortality | Li (2020) |
| Ambient temperature and relative humidity | Impacted on the growth rate of COVID-19 outbreaks | Chaudhuri et al. (2020) |
| Temperature, humidity, and UV-B radiation | Higher transmission risks for COVID-19 | Liu et al. (2020a) |
| Increased temperature and humidity | Partially suppressed COVID-19 incidences | Wu et al. (2020) |
| Air pollutants (PM2.5, PM10, SO2, CO, NO2 and O3) | Short-term exposure to air pollutants (PM2.5, PM10, CO, NO2 and O3) is associated with increased risk of COVID-19 infection; short-term exposure to a higher concentration of SO2 is associated to decreased risk of COVID-19 infection | Zhu et al. (2020) |
5. Safeguards against transmission of droplets and aerosols
The transmission of droplets and aerosols has significant implications on healthcare workers and caretakers managing patients infected with COVID-19, and providing appropriate PPE is, therefore, of utmost importance. The facemasks play a major role in preventing both droplets and aerosols from transmitting the disease from an infected person to a host. Facemasks are popular in controlling and preventing virus transmission, especially in connection with severe respiratory syndromes such as SARS-CoV, MERS-CoV, and SARS-CoV-2, since the absence of any vaccination or specific anti-infective treatments (Long et al., 2020). The surgical mask, N95 respirator, and elastomeric respirator have been popular among many countries with a different degree of success against the COVID-19 virus. Besides, with greater demand for masks in many countries, more sophisticated masks have been experimented by various researchers (Balachandar et al., 2020; Leung and Sun, 2020). Surgical masks and N95 respirators are very popular and ubiquitous among millions of people worldwide as the PPE for COVID-19, but surgical masks are believed to be not preventing aerosol transmission, and N95 respirators are recognized to be preventing aerosol and droplet transmission (Derrick and Gomersall, 2005; Leung et al., 2020; Sandaradura et al., 2020).
The live influenza virus in the air from, in front, and behind all surgical masks have been tested, and the results indicate that a surgical mask will reduce the exposure to aerosolized infectious influenza virus (average 6-fold), depending on the design of the mask (Booth et al., 2013). Another study on masks has manifested that when applied to outpatient healthcare personnel, there was no significant difference in the performances between N95 respirators and medical masks for the incidence of laboratory-confirmed influenza (Radonovich et al., 2019). Long et al. (2020) have corroborated that the use of N95 respirators compared with surgical masks was not associated with a lower risk of laboratory-confirmed influenza. This study pronounced that N95 respirators were not necessary for the general public and non-high risk medical staff those who were not in close contact with influenza patients or suspected patients (Long et al., 2020). Unresolved dichotomy on the route of transmission by virus-laden droplets and aerosols suggested that the use of respirators for healthcare workers against SARS was much advisable than conventional surgical masks that were ineffective against aerosols (Garner, 1996; Wenzel and Edmond, 2003).
With the unexpected escalation of the COVID-19 cases worldwide, there has been a dearth in supply of masks, and consequently, the Center for Disease Control and Prevention, USA, has modified its guidelines on masks with the inclusion of homemade cloth or fabric masks to be worn in public areas. Use of masks can be 2-fold: control the penetration of droplets from an infectious person into the respiratory tract of a susceptible host, and control the droplets going out from an infected patient. Nevertheless, the effectiveness of the use of masks for the control of SARS-CoV-2-laden aerosol transmission from an infected person to a susceptible host is uncertain and not fully conceivable. It has been a known fact that different commercial masks have different efficiencies in controlling the transmission of infectious agents. In general, N95 respirators are provided to prevent users from inhaling small airborne particles (aerosols) and need to fit tightly to the user's face. Surgical masks are often used to protect people from larger droplets transmission and fit loosely to the user's face (Lawrence et al., 2006; Zhiqing et al., 2018).
Complying with European standard EN 149:2001, three different types of disposable particulate respirators known as filtering facepiece 1 (FFP1), FFP2, and FFP3 have been in use for controlling SARS-CoV-2. The FFP1 refers to the least filtering of the three masks with an aerosol filtration of at least 80% and leakage to the inside of a maximum of 22%. This mask is mainly used as a dust mask. The FFP2 masks have a minimum of 94% filtration and a maximum of 8% leakage to the inside. Healthcare professionals often wear them against influenza viruses, believing that they guard against aerosol transmission. The FFP2 masks are also used for protection against the SARS-CoV-2. The FFP3 masks are the best in filtering particles and are recommended against the contraction of SARS-CoV-2. With a minimum filtration of 99% and a maximum 2% leakage to the inside, the FFP3 masks protect the susceptible host against the contraction of the disease caused by very fine particles such as virus-laden aerosols from an infected person.
Another study comparing the efficiency of homemade masks, surgical masks, and standard FFP2 masks has corroborated that surgical masks provided about twice as much protection as homemade masks (van der Sande et al., 2008). The FFP2 masks were observed to provide adults with about 50 times as much protection as homemade masks, and 25 times compared to surgical masks (van der Sande et al., 2008). Similarly, another study has elaborated that a surgical mask (that filtered 89% of viral particles) was about three times better in controlling the viral transmission than that of a homemade mask made of a tee-shirt and cotton towel (Davies et al., 2013). Davies et al. (2013) have further iterated that a homemade mask should only be considered as a last resort to prevent droplet transmission from infected individuals, but with limited success. Elastomeric respirators serve as an alternative to disposable N95 respirator use in healthcare, as both have similar efficiencies in filtering SARS-CoV-2. The primary advantage of elastomeric respirators is the reuse potential with proper cleaning. Leung et al. (2020) have carried out experiments in developing a novel charged PVDF nanofiber filter to capture aerosol particles effectively.
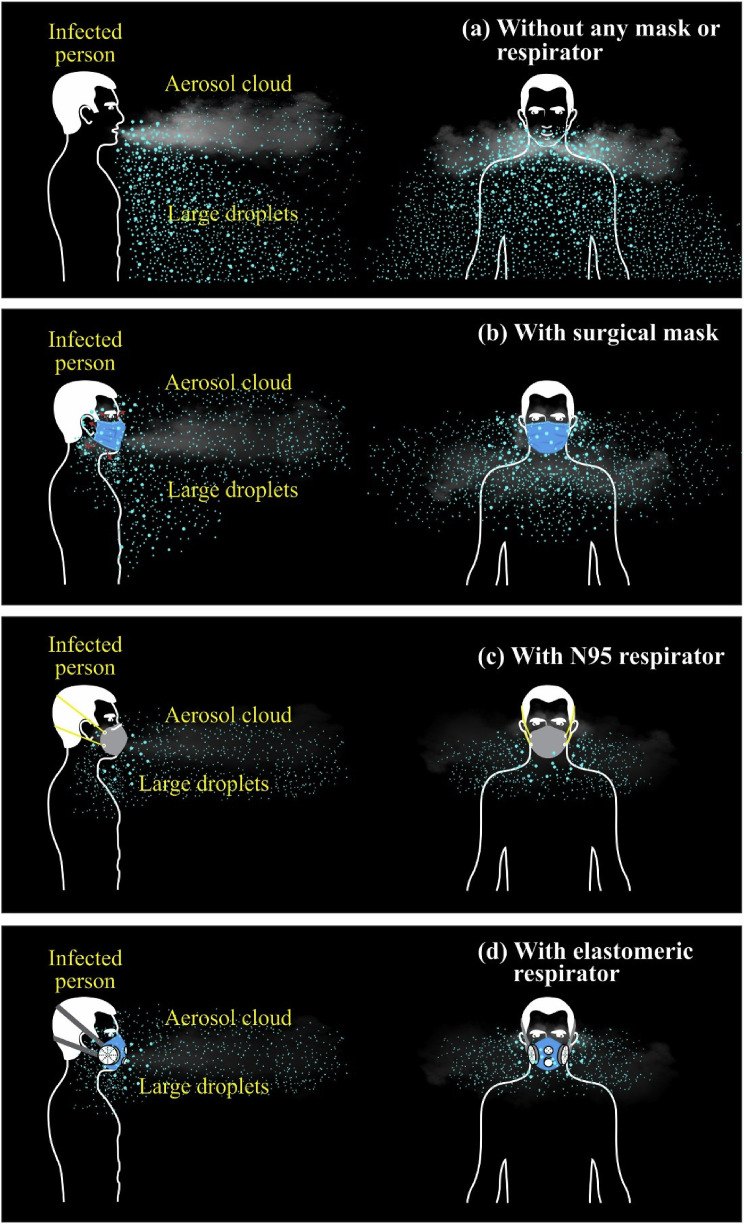
Fig. 3 depicts the trajectories of droplets and aerosols from an infected patient in the event of coughing with different masks and respirators worn. With surgical masks worn, about 20–30% leakage of droplets and a large portion of aerosols, particularly from the loosely fitted sides, could be anticipated (Fig. 3b). With N95 and elastomeric respirators worn, 5% leakage of droplets and a cloud of aerosols could be expected (Fig. 3c and d). None of these masks is guaranteed to cut off SARS-CoV-2 fully; hence, social distancing is vital to be adopted, especially in the indoor environment. With the onset of the COVID-19 pandemic, many researchers have been in the development of effective filtering mechanisms to combat SARS-CoV-2-laden aerosol transmission; however, until early May 2020, there have been no promising PPE developed to curtail such transmission.
Fig. 3.
Trajectories of droplets and aerosols from an infected patient in the event of coughing with different masks and respirators worn (a) without any mask or respirator (b) with surgical mask (c) with N95 respirator (d) with reusable elastomeric respirator.
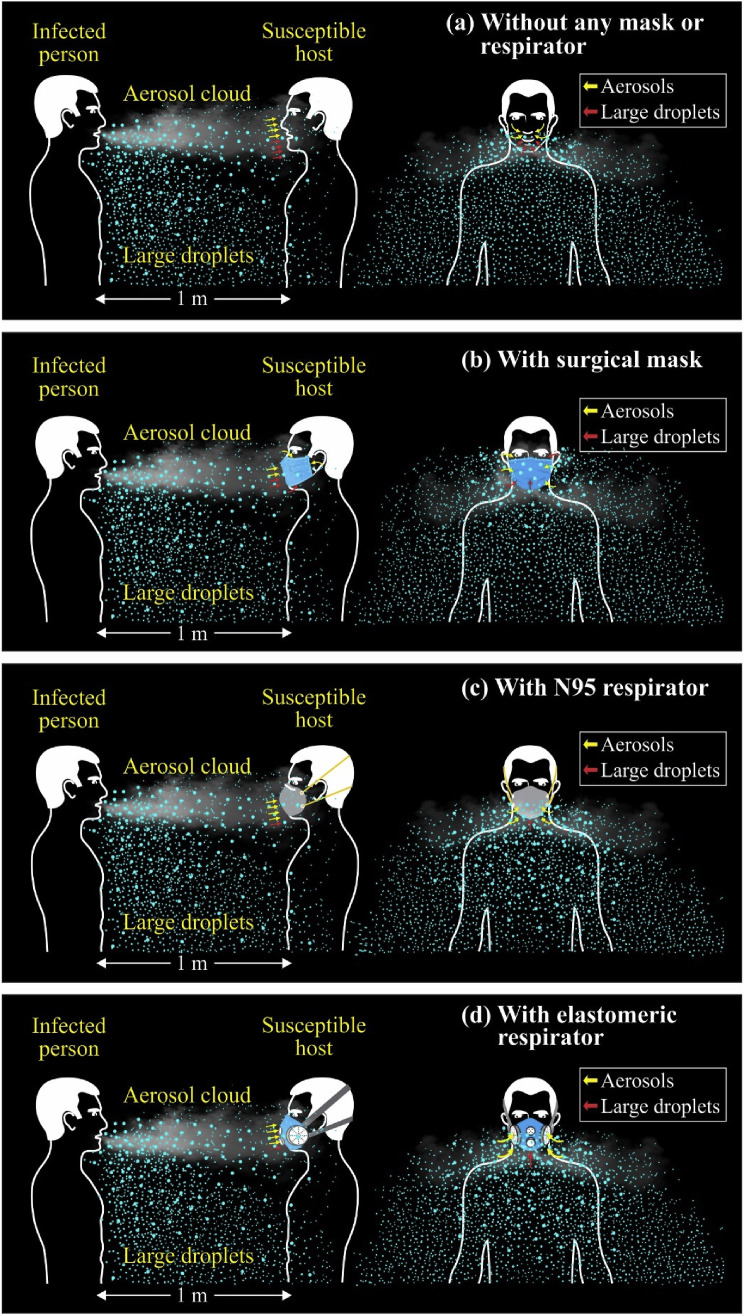
In the meantime, it is imperative to explore situations where an infected patient coughs without any mask worn, and a susceptible host inhales the resultant plume of droplets and aerosols with different masks worn at a distance of 1 m (Fig. 4 ). As shown in Fig. 4a, the host without a mask worn receives a considerable payload of viruses so that it is very likely that he gets infected. However, with a surgical mask worn, he may, during inhalation, filter in 20–30% of the payload of viruses with a lower propensity of getting infected (Fig. 4b). Such a payload may have more than a couple of hundreds of SARS-CoV-2, which is believed to be adequate to instill the COVID-19 among exposed people. The host wearing N95 or reusable elastomeric respirator may not receive in more than 5%, which may, however, constitute more than a few hundreds of payloads of the virus (Fig. 4c and d). The probability of getting infected under such a scenario is still positive, although it is very minute. None of these masks is, however, guaranteed against SARS-CoV-2.
Fig. 4.
Trajectories of droplets and aerosols inhaled by a susceptible host with different masks and respirators worn in the event of coughing by an infected patient (a) without any mask or respirator (b) with surgical mask (c) with N95 respirator (d) with reusable elastomeric respirator.
Part 2:
6. Aerodynamic behavior of SARS-CoV-2-laden droplets and aerosols in different confined spaces
Many people are reported to contract the COVID-19 in confined spaces. Thus, it is worthwhile to describe how such phenomena help intensify the mass occurrence of the COVID-19 in different confined spaces under varying microclimatic conditions. In this respect, three confined spaces such as inside the cabin of an airplane, interior space of a car, and common dormitory-type space of a healthcare or isolation center were selected.
6.1. Airplane cabin
Since over two billion people travel on commercial flights each year (Silverman and Gendreau, 2009), the behavior of SARS-CoV-2 in the cabin is paramount to be understood. Air travelers spend extended periods in enclosed spaces, even for more than 10 h, which usually facilitates a conducive environment for the spread of infectious diseases. Extensive aerodynamic modeling has been performed to get an insight into how the buoyant jet of coughing by an infected person of a respiratory illness spreads in the cabin of a flight (Redrow et al., 2011; Yang et al., 2017). The hypothesis on the most affected zones within the cabin is, therefore, highlighted below.
The cabin of a flight is usually provided with airflow from cabin air outlets and individual outlets located in the overhead compartment that runs the length of the cabin. A sheet of airflow typically in the form of a jet with lower temperatures (< 25 °C) is projected down, and finds its way towards the bottom of the cabin (return air grills located on the sidewalls) from which it goes to the underfloor area. However, looking at a more detailed picture, there are two typical airflow fields developed (Fig. 5 a). The first zone called the jet zone, established in the upper deck areas of the cabin, is characterized in terms of large-scale circulations, while the collision zone found in the middle and lower floor area is characterized by interactions of two lateral jets (Li et al., 2017) (Fig. 5a). In general, about 3.6–7.4 L/s of air per passenger is provided, of which half of the volume is the filtered and recirculated air, and the other half is outside air (Bagshaw and Illig, 2019). Such an arrangement brings in a complete cabin air exchange every two to 3 min (20–30 air changes per hour (ACH)) (Bagshaw and Illig, 2019). The high air exchange rate controls the temperature gradients, prevents stagnant cold areas, maintains air quality, and dissipates payloads of virus-laden droplets and aerosols. In a typical aircraft, the recirculated air is passed through high-efficiency particulate air (HEPA) filters, with which in excess of 99.97% of particles characterized by aero-diameter of 0.3 μm could be removed from the ingress of cabin air. Exhaled droplets and aerosols from passengers and crew often increase the humidity to an average of 6–10%, which is below the 20% normally accepted as comfort level (de Ree et al., 2000).
Fig. 5.
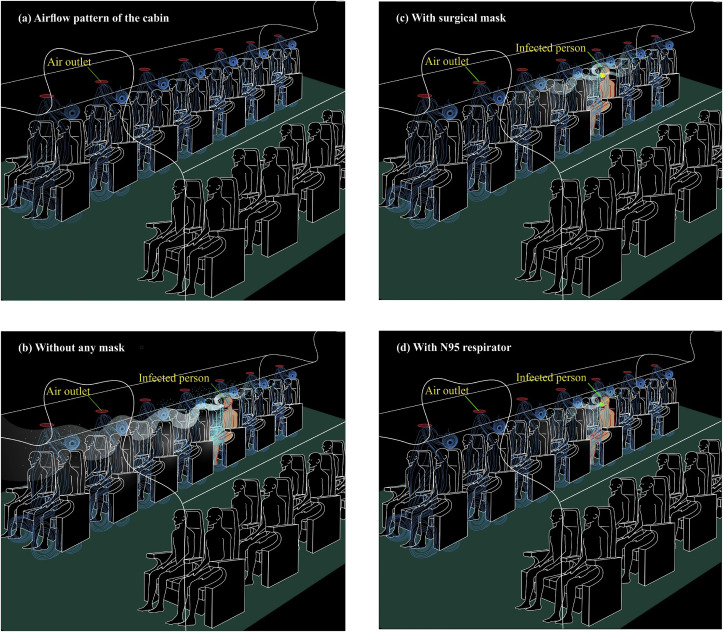
Trajectories of droplets and aerosols from an infected patient in the event of coughing in an aircraft (a) airflow pattern of the cabin without any cough-jet expiration (b) without any mask (c) with surgical mask (d) with N95 respirator.
In the flight cabins, because of the densely packed environment, the cough-jet released by a SARS-Cov-2 infected person is expected to break the local airflow, particularly the jet zone, and travels both forward and backward directions in the proximity of the point of exit (Fig. 5b). Since the velocity of exiting the violent expiration (coughs) is around 10 m/s, the droplets may travel four to five seats ahead, and the aerosol-cloud could go even further away (Fig. 5b). However, there is no lateral movement expected except the immediate passenger on either side. In contrast to the forward movement, there is a backward movement of droplets typically by one seat, but the aerosol movement may be more. This phenomenon illustrates that about five to ten people could get infected with the disease with an infected person onboard. Nevertheless, the propensity of getting sick by exposure to a plume of aerosols produced by cough-jet is poorly understood, and the actual number of contracted cases may be far from recorded. The Brownian motion followed by airflow jet movement governs the aerosol plume, after the dissipation of advective transport. Such movement supports an agglomeration of virus-laden aerosols in fomites at passenger levels. It is, therefore, crucial to decide by all airlines that such suspicious fomites such as papers, magazines, pillows, and blankets be disposed of perhaps subjected to thermal destruction until the COVID-19 pandemic recedes.
Fig. 5c illustrates how the cough-jet trajectory travels with the patient equipped with a surgical mask. With the surgical mask worn, the droplets are meant to travel up to one-two seats forward, and one seat backward. Such phenomena maybe because of the jet coming out from either side of the mask, as the mask is not tight enough on both sides. Nevertheless, the aerosol cloud will travel far from two seats front and one seat behind by the Brownian motion coupled with the airflow trajectories of the cabin. The streamlines of airflow are usually directed downward so that there will be a contribution of virus-laden aerosols back to the people on board. The illustration in Fig. 5d is more or less the same as that of 5c, with the exception that both droplets and aerosols do not travel far. With the N95 mask worn, an infected patient sheds droplets forward and backward by one seat and more than one seat for aerosols. The behavior of virus-laden aerosols resulted from a cough-jet has not yet been aerodynamically modeled with reasonable accuracy; hence, the actual level of impact that a single cough-jet envisages could not be simulated well. However, there exists evidence to showcase a profound risk of COVID-19 being spread in an aircraft when a symptomatic or even asymptomatic patient is on board. Further, the environmental factors such as moderately low relative humidity (50%), low temperature (< 25 °C), and moderate ACH (< 30 per hour) would set the platform for the SARS-CoV-2 to sustain for extended periods within the cabin. Strict guidelines for the minimization of such pandemic events are, therefore, paramount.
6.2. Passenger car
International Organization of Motor Vehicle Manufacturers (OICA) has estimated that over 1 billion passenger cars travel on roads by 2019 worldwide, indicating that one out of seven people of the world has a passenger car. When the world is open back to normalcy by lifting the present state of lockdown, people will resort to traveling by passenger cars, and consequently, there will be a propensity of spreading the COVID-19 unless precautions are taken. We, therefore, bring in a hypothesis to illustrate the best possible ways of preventing the COVID-19 from spreading while traveling in a passenger car.
A crucial attribute that supports the spread of COVID-19 is the interior ventilation rate in the passenger vehicle, usually expressed in ACH, which depends on the vehicular speed, ventilation setting and window positions (Ott et al., 2007). Engelmann et al. (1992) have estimated that with the air-conditioning (AC) system off, the ACH for a stationary vehicle was in the range of 0.42–1.09 per hour. With the AC on, ACH was between 1.96 and 3.23 per hour, and with the AC off and the fans on, it varied in the range of 8.7–10.7 per hour. Park et al. (1998), with the windows closed and no mechanical ventilation, have reported the ACH between 1.0 and 3.0 per hour, and with the ventilation set on recirculation, between 1.8 and 3.7 per hour. With the windows closed and the fan set on fresh air, the ACH was between 13.3 and 26.1 per hour, and with windows open, but no mechanical ventilation, the ACH ranged from 36.2 to 47.5 per hour (Park et al., 1998). Offermann et al. (2002) have measured the ACH by letting the vehicle move with an average speed of 29 km/h and have found that with the window open and the ventilation system off, an ACH of 71 per hour, with the ventilation system on and the windows closed, 60 per hour, and when the ventilation system was turned off, 4.9 per hour.
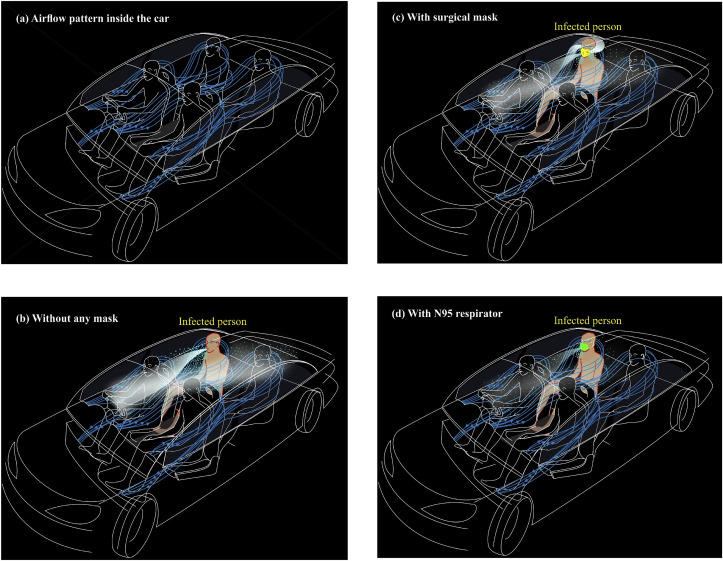
Following the study done by Khatoon and Kim (2020), a typical pattern of velocity streamlines inside the vehicular cabin with a moderate level of ACH assigned to a vehicle moving at a moderate speed under conditions of “AC on and windows closed” is shown in Fig. 6 a. Fig. 6a illustrates that cooled air travels to the back seats and returns towards the front on either side at a lower level. Under such circumstances, an infected person sitting in the back seat may cough and the resultant cough-jet in the form of droplets and a plume of aerosols (with an average speed of 10 m/s; relative humidity < 50%; temperature < 25 °C; ACH < 60 per hour) spreads towards the front seat, and the plume of aerosols may drop the advective transport phenomena with lower velocities and get carried away with existing velocity streamlines once again towards the back seats (Fig. 6b). Such phenomena may expose all passengers in the vehicle, and the risk of contracting the disease seems to be high. Two such cases have been reported in Sri Lanka, where an infected passenger had travelled sitting at the back seat in a rented car for a period not greater than 1 h with AC on and windows closed, and the driver was subsequently reported to have got infected of the COVID-19. The other case was reported that a person had accompanied one of his siblings (an asymptomatic person) in his car with AC on and windows closed for more than 15 min. Such situations seem to be somewhat controlled when the infected person wears a surgical mask. However, the risk factor remains the same, as loose ends of the mask shed both droplets and aerosols, although the expiration from the front of the mask is substantially reduced (Fig. 6c). Conversely, when the infected passenger is equipped with an N95 respirator, under the same conditions, a minute payload of droplets and a faint cloud of aerosols may come out (Fig. 6d). However, because of the circulation within the cabin, one cannot rule out that there is no element of risk. Thus, a hypothesis could be built speculating that traveling in a passenger vehicle with people aboard under conditions of AC on and window closed, has a discernible risk factor of getting susceptible hosts infected, though masks are worn.
Fig. 6.
Trajectories of droplets and aerosols from an infected patient in the event of coughing in a car with air-conditioner switched on (a) airflow pattern inside the car without any cough-jet expiration (b) without any mask (c) with surgical mask (d) with N95 respirator.
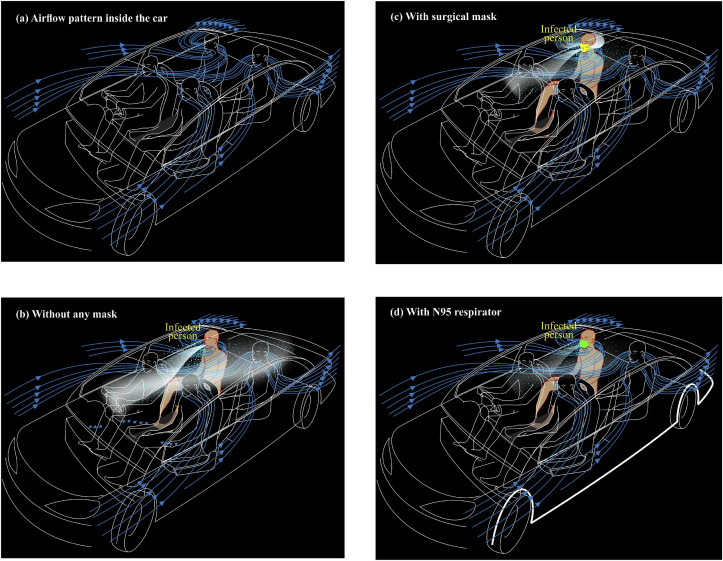
When a passenger car moves at a certain speed with windows open, the velocity streamlines are generated from front and rear windows, and finally, sweeping the passengers aboard, they exit the cabin from the rear windows (Fig. 7 a). Such transport-phenomena are simulated using computational fluid dynamics, but detailed information on the behavior of streamlines under different environmental settings is poorly investigated. In the case of passenger cars with windows open, different behaviors could be expected depending on the environmental settings prevailing in different geographical regions. In other words, the environmental settings for temperate climates such as East Asia, Europe, and North America (relative humidity < 50%; temperature < 25 °C; ACH > 60 per hour) and tropical climates, including South East Asia, Africa, and South America (relative humidity > 50%; temperature > 25 °C; ACH > 60 per hour) could be expected. The studies done on the sustenance of SARS-CoV-2 have manifested that there may be a better chance for the viral-laden cough-jets to sustain in temperate climates than tropical climates, as the daily mortality of COVID-19 has been positively associated with diurnal temperature range, but negatively with the absolute humidity (Ma et al., 2020).
Fig. 7.
Trajectories of droplets and aerosols from an infected patient in the event of coughing in a car with windows opened (a) airflow pattern inside the car without any cough-jet expiration (b) without any mask (c) with surgical mask (d) with N95 respirator.
Fig. 7b shows how the cough-jet behaves in a passenger car with windows open and AC off when the car moves at a speed of less than 30 km/h. Under such conditions, the droplets fall in the entire length of the vehicle, while the aerosol-cloud drives to the front and returns with the airflow streamlines, spreading the aerosol plume every part of the cabin in no time. When the car moves at higher speeds (> 30 km/h) with the same environmental settings, the droplets do not travel far and confined to a limited space (even not beyond the driver's seat), but the cloud of aerosol will drift far and finally exits from the rear windows. The explanations given in this paper restrict the analysis only for the case where the speed is less than 30 km/h, as such speeds become the worse scenario for the sustenance of the SARS-CoV-2 virus.
The cabin environment becomes much improved when the infected person wears a surgical mask while traveling (Fig. 7c). There seems that only a minimal payload of droplets being shed from the front, but considerable load may come from either side of the mask, as the surgical mask is usually loosely fitted to the face. Conversely, the aerosol cloud may still travel to the front area of the cabin and returns with the airflow stream coming from outside the vehicle. Nevertheless, the cabin airflow streamlines drive such virus-laden plume out of the cabin in seconds. The cabin environment is further improved when the infected person wears an N95 respirator (Fig. 7d). Still, one has to admit the fact that there is an element of risk for susceptible hosts to get infected.
When two scenarios (Scenario 1: AC on and windows closed; Scenario 2: AC off and windows opened) are critically reviewed, one can speculate that the scenario 2 will be better in controlling the SARS-CoV-2 virus; hence strongly recommended at least until the COVID-19 pandemic ceases. For example, the second patient of COVID-19 in Sri Lanka was a tour guide, and when he became symptomatic, he travelled to the hospital by his car driven by his son, with his wife sitting in the front seat. He made it a point to open all windows and sat behind until they reached the hospital. The traveling time was more than 30 min, and no person in the car was infected with the COVID-19. This story epitomizes the rationale postulated above, and the relevant authorities of affected countries should come out with strict guidelines to get such best practices implemented for reduced morbidities and mortalities. Conversely, two cases were reported in Sri Lanka, where drivers of rental cars got infected with scenario 1. Besides, letting the car park under direct sunlight with windows open for at least 30 min would be a better option to eradicate the potential payloads of the SARS-CoV-2 virus from the cabins of passenger cars.
6.3. Healthcare center
It would be imperative to explore the plausible factors of transmitting SARS-CoV-2 virus within indoor spaces, preferably makeshift hospitals, and healthcare, quarantine and isolation centers where accommodation facilities have large open spaces with many beds laid in a sequence. Such a facility is, in this paper, described in respect of a healthcare center, but could be applicable for other indoor spaces mentioned above. It is a known fact that the SARS diseases became epidemic and sometimes pandemic, forcing the authorities seek isolation facilities beyond their usual capacities available. Such gestures invariably drive the authorities to build appropriate healthcare centers or convert other existing facilities in a short period. Such spaces often become large floor areas whose ventilation facilities maybe poor in cleaning the virus-laden airborne plumes. The transmission of SARS diseases in an epidemic or pandemic situation is usually 2-fold. The first being the non-nosocomial transmission by which suspected patients from outside will be brought into the healthcare center. In addition, with time, susceptible hosts residing at healthcare centers will contract the disease through nosocomial transmission unless the ventilation facilities (> 6 ACH or 1.6 L/s/m3, negative pressure difference > 2.5 Pa, and the airflow difference > 56 L/s) are adequate (WHO, 2009). The differentiation of both these transmission modes for a given situation is, however, a daunting task and extremely difficult (Bi et al., 2007).
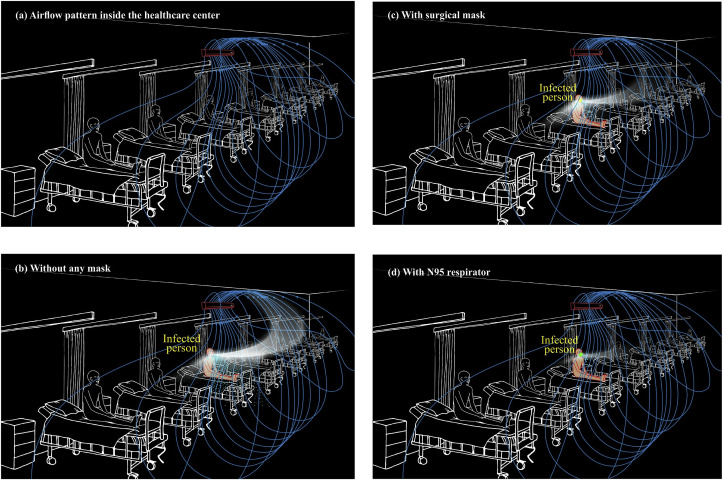
In a confined space of a healthcare center, appropriate management of non-nosocomial transmission should be implemented to control the onset of nosocomial transmission, where ventilation methods play a vital role. Given the fact that inadequate ventilation prevails in a confined space, another classification indicates that 2-fold transmission types are distinguished; short-range (between individuals, generally less than 1-m apart) and long-range (within a room, between rooms or between distant locations, generally greater than 1-m distances) (Tang et al., 2006). Expiration of cough-jets of an infected person composed of droplets and aerosols enters and mixes with air in the breathing zone of a susceptible host standing nearby (e.g., medical staff), which is capable of contracting the disease (short-range transmission) between individuals may interact to infect one another. In the meantime, cough-jet travels long distances depending on the airflow pattern of the space through the aerosol plume (long-range transmission) contracting people a couple of meters away from the infected person. The airflow in the confined space is often governed by a combination of differences in temperatures and humidity. Fig. 8 a illustrates the airflow patterns of an open area equipped with a series of beds meant for suspected patients with AC on and all openings closed for a tropical climate. Airflow streamlines are first generated by the AC and pushed down sweeping the patients, and once the advective velocities diminish, airflow mass starts moving up through convective currents, as the temperature becomes hotter. The hot air will then be extracted by the AC and cleaned through a filter before sending back to the same space.
Fig. 8.
Trajectories of droplets and aerosols from an infected patient in the event of coughing in a healthcare center with ventilation provided by an air conditioner (a) airflow pattern inside the healthcare center without any cough-jet expiration (b) without any mask (c) with surgical mask (d) with N95 respirator.
Fig. 8b manifests a typical pattern of a cough-jet trajectory of an infected person in the healthcare center with the provision of an AC driven airflow. The droplets fall within a short distance, creating an environment conducive for short-range transmission of SARS-CoV-2-laden droplets. However, the virus-laden aerosol plume travels far from the immediate neighborhood and gets airborne with the convective currents developed within the confined space (Fig. 8b). Such aerosol plume developed could follow the airflow trajectories, which are often altered by moving objects, opening and closing of doors and windows, and temperature and humidity variations. Besides, a certain fraction of the virus-laden aerosols will diffuse towards lateral directions by Brownian motion resulting in nosocomial transmission to many susceptible hosts in the same confined room (not shown in Fig. 8b). These aerosol-generating plumes cause long-range transmission within the confined space, contracting many susceptible hosts far more than one could imagine.
Fig. 8c shows the cough-jet trajectory with the infected patient wearing a surgical mask. With the surgical mask worn, the payload of droplets from the infected patient reduces drastically and restricted to a small distance. The neighboring people on either side may not be exposed to direct contamination, but they could contract the disease by touching fomites-laden viruses. However, virus-laden aerosols will travel forward and disappear via convective and diffusion processes. Such transport phenomena may carry the disease-causing viral loads, promoting nosocomial infection. A similar scenario is observed with a patient wearing an N95 respirator, but to a lesser extent compared to that of a surgical mask (Fig. 8d). The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (2020) claims that although the nosocomial transmission of SARS-CoV-2 by coughing is still unclear, 1,716 healthcare workers in makeshift hospitals in China have been infected by February 11, 2020. Such massive numbers of infections, even with appropriate PPE worn by healthcare workers, may have been propelled by the nosocomial transmission of airborne SARS-CoV-2-laden aerosols that have lingered for many hours because of poor mixing ventilation. Lu et al. (2020) have reported an AC-propelled COVID-19 infection of a host by an asymptomatic patient in a restaurant in Guangzhou, China, indicating a likelihood of airborne transmission by poor mixing ventilation. The CDC in a press conference has intimated that as of April 09, 2020, about 9,000 healthcare workers in the USA have shown positive results for the COVID-19 test, which could have been because of nosocomial transmission caused by airborne aerosol clouds.
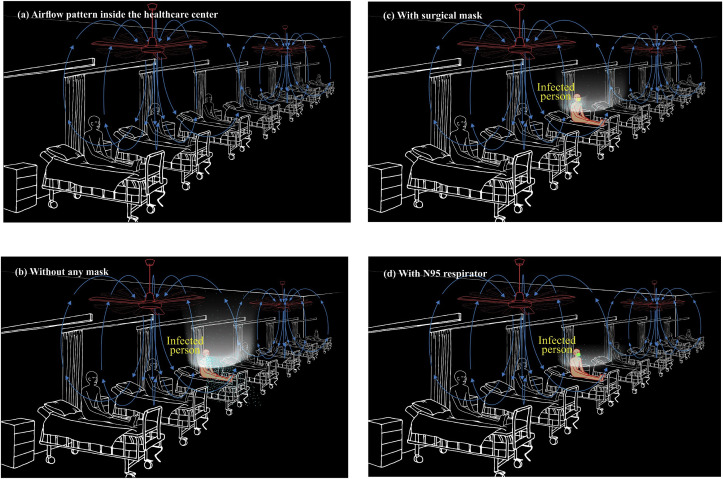
The mixing ventilation driven by ceiling-held mechanical fans is also popular among developing countries, particularly of tropical regions. For example, Sri Lanka has converted many confined spaces belonging to the military forces of the country to be mobilized as quarantine centers. Fig. 9 a shows a typical arrangement of such a space where ventilation is provided by ceiling fans. It is found that the people who are quarantined are given bed facilities at a distance of 1 m between each other, as shown in Fig. 9a. The mechanical ventilation propelled by ceiling fans generates downdraft airflow with an advective force, and it sweeps against people in the confined space. As the airflow passes the people and objects in the confined area, it becomes warmer and starts moving up through the process of convection (Fig. 9a). Such warm air travels upward the ceiling and, perhaps, exits from openings. If there are no adequate openings, poor indoor ventilation sustains, and such warm air may fill in the whole confined space resulting in nosocomial infection by SARS-CoV-2-laden plumes. Fig. 9b shows such an occurrence where droplets and a plume of aerosols being shed by an infected person. Fig. 9c and d illustrate environmental settings with the infected person equipped with a surgical mask and an N95 respirator, respectively. Under both cases, droplet transmission seems to be subdued to a greater extent, but the virus-laden aerosol transmission will be plausible. The common scenarios of healthcare centers such as inadequate openings restricting the fresh air ingress and exit, not having an adequate number of fans to impart acceptable ACH, a large number of people living in such a facility, and inadvertent blockages of air paths by people's belongings, equipment, and movements, among other things may cause poor ventilation in the environmental settings and trigger COVID-19 outbreak through the nosocomial transmission. Sri Lanka reports that as of April 30, 2020, in a Naval Complex in Colombo, there have been more than 150 sailors contracted with the COVID-19. The sailors have been on duty in cordoning off of potential areas of COVID-19 pandemic. However, it has been brought to the notice that when they returned to the base, many of them have stayed in confined areas whose ventilation potential driven by mechanical fans was rather poor. This scenario has been a classic example of the airborne infection caused by poor ventilation that has promoted the virus-laden aerosol plume to linger for many hours inside the building.
Fig. 9.
Trajectories of droplets and aerosols from an infected patient in the event of coughing in a healthcare center with ventilation provided by ceiling fans (a) airflow pattern inside the healthcare center without any cough-jet expiration (b) without any mask (c) with surgical mask (d) with N95 respirator.
Taking all case studies mentioned above into consideration, one cannot simply ignore that both droplet and aerosol laden transmissions of COVID-19 are uncertain; hence administrative, clinical, and physical best management practices are paramount in implementing, especially in confined spaces.
7. Conclusion
Researchers have speculated that both droplets and aerosols generated from non-violent and violent expirations of SARS-CoV-2-infected people may be responsible for the airborne transmission of COVID-19 disease. However, more research work should be conducted to understand the behavior of virus-laden droplets and aerosols in different environmental settings, especially confined spaces so that the transmission of COVID-19 pandemic in the built environment could be fully ascertained. The case studies found worldwide indicate that the behavior of the SARS-CoV-2 virus has been unprecedentedly unique with more survival and viable rates in the air and believed to linger in the air for an extended period. The challenge before many healthcare workers in combatting the disease would be a daunting task unless proper administrative, clinical, and physical measures are taken within the healthcare settings. Inter-disciplinary research on the behavior of the SARS-CoV-2 virus needs to be conducted to prevent COVID-19 disease from spreading worldwide.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Mahesh Jayaweera: Conceptualization, Methodology, Investigation, Writing - original draft, Visualization, Supervision, Project administration. Hasini Perera: Conceptualization, Methodology, Resources, Validation, Formal analysis, Investigation, Data curation, Writing - original draft. Buddhika Gunawardana: Validation, Resources, Writing - review & editing. Jagath Manatunge: Validation, Resources, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors wish to acknowledge the assistance rendered by many in collating information on COVID-19 case studies.
References
- Bagshaw M., Illig P. Travel Med. Elsevier; 2019. The aircraft cabin environment; pp. 429–436. [DOI] [Google Scholar]
- Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandar V., Mahalaxmi I., Kaavya J., Vivekanandhan G., Ajithkumar S., Arul N., Singaravelu G., Kumar N.S., Devi S.M. COVID-19: emerging protective measures. Eur. Rev. Med. Pharmacol. 2020;24:3422–3425. doi: 10.26355/eurrev_202003_20713. [DOI] [PubMed] [Google Scholar]
- Baron P.A., Willeke K. second ed. van Nostrand Reinhold; New York: 2001. Aerosol Measurement: Principles, Techniques, and Applications. [Google Scholar]
- Beggs C.B. Is there an airborne component to the transmission of COVID-19?: a quantitative analysis study. medRxiv. 2020 doi: 10.1101/2020.05.22.20109991. [DOI] [Google Scholar]
- Bi P., Wang J., Hiller J.E. Weather: driving force behind the transmission of severe acute respiratory syndrome in China? Intern. Med. J. 2007;37:550–554. doi: 10.1111/j.1445-5994.2007.01358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P.K., Islam M.Z., Debnath N.C., Yamage M. Modeling and roles of meteorological factors in outbreaks of highly pathogenic avian influenza H5N1. PloS One. 2014;9 doi: 10.1371/journal.pone.0098471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone S.A., Gerba C.P. Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 2007;73:1687–1696. doi: 10.1128/aem.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C.M., Clayton M., Crook B., Gawn J.M. Effectiveness of surgical masks against influenza bioaerosols. J. Hosp. Infect. 2013;84:22–26. doi: 10.1016/j.jhin.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Bourouiba L. Images in clinical medicine: a sneeze. N. Engl. J. Med. 2016;375:e15. doi: 10.1056/NEJMicm1501197. [DOI] [PubMed] [Google Scholar]
- Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.4756. [DOI] [PubMed] [Google Scholar]
- Bourouiba L., Dehandshoewoercker E., Bush J.W.M. Violent respiratory events: on coughing and sneezing. J. Fluid Mech. 2014;745:537–563. doi: 10.1017/jfm.2014.88. [DOI] [Google Scholar]
- Brankston G., Gitterman L., Hirji Z., Lemieux C., Gardam M. Transmission of influenza A in human beings. Lancet Infect. Dis. 2007;7:257–265. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- Burke R.M. MMWR; 2020. Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19—United States, January–February 2020. Morbidity and mortality weekly report, Centers for Disease Control and Prevention (CDC). 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova L.M., Jeon S., Rutala W.A., Weber D.J., Sobsey M.D. Effects of air temperature and relative humidity on coronavirus survival on surfaces. Appl. Environ. Microbiol. 2010;76:2712–2717. doi: 10.1128/aem.02291-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2003. Guidelines for Environmental Infection Control in Health-Care Facilities.https://www.cdc.gov/infectioncontrol/guidelines/environmental/index.html (Updated: July 2019) Morbidity and Mortality Weekly Report, 52 (RR-10) [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2020. Coronavirus Disease 2019 (COVID-19): How COVID-19 Spreads.https://www.cdc.gov/coronavirus/2019-ncov/prepare/transmission.html [Google Scholar]
- Centers for Disease Control and Prevention (CDC) 2020. Travelers from Countries with Widespread Sustained (Ongoing) Transmission Arriving in the United States.https://www.cdc.gov/coronavirus/2019-ncov/travelers/after-travel-precautions.html [Google Scholar]
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., Tsoi H.W. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Peiris J.S., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv. Virol. 2011;2011:1–7. doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri S., Basu S., Kabi P., Unni V.R., Saha A. 2020. Modeling Ambient Temperature and Relative Humidity Sensitivity of Respiratory Droplets and Their Role in Determining Growth Rate of Covid-19 Outbreaks.arXiv:2004.10929 [Google Scholar]
- Chen C., Zhao B. Makeshift hospitals for COVID-19 patients: where health-care workers and patients need sufficient ventilation for more protection. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/s0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell G., Towers S., Viboud C., Fuentes R., Sotomayor V., Simonsen L., Miller M.A., Lima M., Villarroel C., Chiu M., Villarroel J.E. The influence of climatic conditions on the transmission dynamics of the 2009 A/H1N1 influenza pandemic in Chile. BMC Infect. Dis. 2012;12:298. doi: 10.1186/1471-2334-12-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C.S. Physical aspects of bioaerosols particles. In: Cox C.S., Wathes C.M., editors. Bioaerosols Handbook. Lewis Publishers; Boca Raton, FL, USA: 1995. pp. 15–25. [Google Scholar]
- Darnell M.E.R., Subbarao K., Feinstone S.M., Taylor D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome. SARS-CoV. J. Virol. Methods. 2004;121:85–91. doi: 10.17632/10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A., Thompson K.A., Giri K., Kafatos G., Walker J., Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med. Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ree H., Bagshaw M., Simons R., Brown R.A. Ozone and relative humidity in airliner cabins on polar routes: measurements and physical symptoms. In: Nagda N.L., editor. Air Quality and Comfort in Airliner Cabins. ASTM International; West Conshohocken, PA: 2000. pp. 243–258. [DOI] [Google Scholar]
- Derrick J.L., Gomersall C.D. Protecting healthcare staff from severe acute respiratory syndrome: filtration capacity of multiple surgical masks. J. Hosp. Infect. 2005;59:365–368. doi: 10.1016/j.jhin.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S.M., Zhao X.S., Wen R.F., Huang J.J., Pi G.H., Zhang S.X., Han J., Bi S.L., Ruan L., Dong X.P. Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomed. Environ. Sci.: BES. 2003;16:246–255. [PubMed] [Google Scholar]
- Duguid J.P. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinb. Med. J. 1945;52:385–401. [PMC free article] [PubMed] [Google Scholar]
- Engelmann R.J., Pendergrass W.R., White J.R., Hall M.E. The effectiveness of stationary automobiles as shelters in accidental releases of toxic materials. Atmos. Environ. 1992;26:3119–3125. doi: 10.1016/0960-1686(92)90469-2. [DOI] [Google Scholar]
- Garner J.S., Hospital Infection Control Practices Advisory Committee Guideline for isolation precautions in hospitals. Infect. Control Hosp. Epidemiol. 1996;17:53–80. doi: 10.2307/30142367. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A.E. Severe acute respiratory syndrome-related coronavirus–the species and its viruses, a statement of the coronavirus study group. BioRxiv. 2020:1–15. doi: 10.1101/2020.02.07.937862. [DOI] [Google Scholar]
- Gralton J., Tovey E., McLaws M.L., Rawlinson W.D. The role of particle size in aerosolised pathogen transmission: a review. J. Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson S.A., Griffiths P.S., Perez M.K., Piedimonte G. Detection of airborne respiratory syncytial virus in a pediatric acute care clinic. Pediatr. Pulmonol. 2016;52:684–688. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Gupta D. 2020. Effect of Ambient Temperature on COVID-19 Infection Rate. Available at SSRN 3558470. [DOI] [Google Scholar]
- Harper G.J. Airborne micro-organisms: survival tests with four viruses. J. Hyg. 1961;59:479–486. doi: 10.1017/s0022172400039176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmes J.H., Winkler K., Kool S.M. Virus survival as a seasonal factor in influenza and poliomyelitis. Antonie Leeuwenhoek. 1962;28:221–233. doi: 10.1007/bf02538737. [DOI] [PubMed] [Google Scholar]
- Hinds W.C. second ed. John Wiley & Sons; Hoboken, New Jersey, USA: 1999. Aerosol Technology: Properties, Behavior, and Measurement of Air Borne Particles. [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D.S., Chan P.K. Severe acute respiratory syndrome and coronavirus. Infect. Dis. Clin. North Am. 2010;24:619–638. doi: 10.1016/j.idc.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz M.K., Brunner A.H., Sattar S.A., Nair R.C., Johnson-Lussenburg C.M. Survival characteristics of airborne human coronavirus 229E. J. Gen. Virol. 1985;66:2743–2748. doi: 10.1099/0022-1317-66-12-2743. [DOI] [PubMed] [Google Scholar]
- Judson S.D., Munster V.J. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11:940. doi: 10.3390/v11100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karapiperis C., Kouklis P., Papastratos S., Chasapi A., Ouzounis C. 2020. Assessment for the Seasonality of Covid-19 Should Focus on Ultraviolet Radiation and Not ‘warmer Days’. LttE, v9, 28-Apr-2020 – osf.io. [Google Scholar]
- Khatoon S., Kim M.H. Thermal comfort in the passenger compartment using a 3-D numerical analysis and comparison with Fanger's comfort models. Energies. 2020;13:690. doi: 10.3390/en13030690. [DOI] [Google Scholar]
- Kim S.W., Ramakrishnan M.A., Raynor P.C., Goyal S.M. Effects of humidity and other factors on the generation and sampling of a coronavirus aerosol. Aerobiologia. 2007;23:239–248. doi: 10.1007/s10453-007-9068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormuth K.A., Lin K., Prussin A.J., Vejerano E.P., Tiwari A.J., Cox S.S., Myerburg M.M., Lakdawala S.S., Marr L.C. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. J. Infect. Dis. 2018;218:739–747. doi: 10.1093/infdis/jiy221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Morawska L. Could fighting airborne transmission be the next line of defence against COVID-19 spread? City Environ. Interact. 2019;4 doi: 10.1016/j.cacint.2020.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok Y.L.A., Gralton J., McLaws M.L. Face touching: a frequent habit that has implications for hand hygiene. Am. J. Infect. Contr. 2015;43:112–114. doi: 10.1016/j.ajic.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R.B., Duling M.G., Calvert C.A., Coffey C.C. Comparison of performance of three different types of respiratory protection devices. J. Occup. Environ. Hyg. 2006;3:465–474. doi: 10.1080/15459620600829211. [DOI] [PubMed] [Google Scholar]
- Leung N.H., Chu D.K., Shiu E.Y., Chan K.H., McDevitt J.J., Hau B.J., Yen H.L., Li Y., Ip D.K., Peiris J.M., Seto W.H. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020;1–5 doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W.W.F., Sun Q. Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Separ. Purif. Technol. 2020 doi: 10.1016/j.seppur.2020.116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Liu J., Wang C., Wesseling M., Müller D. PIV experimental study of the large-scale dynamic airflow structures in an aircraft cabin: swing and oscillation. Build. Environ. 2017;125:180–191. doi: 10.1016/j.buildenv.2017.07.043. [DOI] [Google Scholar]
- Li K. The link between humidity and COVID-19 caused death. J. Biosci. Med. 2020;8:50–55. https://.org/10.4236/jbm.2020.86005 [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S., Lau E.H., Wong J.Y., Xing X. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Wang S., Zhang Y., Zhong R. 2020. Relationship between the COVID-19 Outbreak and Temperature, Humidity, and Solar Radiation across China.https://ssrn.com/abstract=3594115 Available at SSRN: [DOI] [Google Scholar]
- Liu J., Liao X., Qian S., Yuan J., Wang F., Liu Y., Wang Z., Wang F.S., Liu L., Zhang Z. Community transmission of severe acute respiratory syndrome Coronavirus 2, Shenzhen, China, 2020. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wei J., Li Y., Ooi A. Evaporation and dispersion of respiratory droplets from coughing. Indoor Air. 2016;27:179–190. doi: 10.1111/ina.12297. [DOI] [PubMed] [Google Scholar]
- Loh N.H.W., Tan Y., Taculod J., Gorospe B., Teope A.S., Somani J., Tan A.Y.H. The impact of high-flow nasal cannula (HFNC) on coughing distance: implications on its use during the novel coronavirus disease outbreak. Can. J. Anesth. 2020;1–2 doi: 10.1007/s12630-020-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y., Hu T., Liu L., Chen R., Guo Q., Yang L., Cheng Y., Huang J., Du L. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta‐analysis. J. Evid. Base Med. 2020 doi: 10.1111/jebm.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen A.C., Mubareka S., Steel J., Palese P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007;3:e151. doi: 10.1371/journal.ppat.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen A.C., Steel J., Mubareka S., Palese P. High temperature (30 C) blocks aerosol but not contact transmission of influenza virus. J. Virol. 2008;82:5650–5652. doi: 10.1128/jvi.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Gu J., Li K., Xu C., Su W., Lai Z., Zhou D., Yu C., Xu B., Yang Z. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhao Y., Liu J., He X., Wang B., Fu S., Yan J., Niu J., Zhou J., Luo B. Effects of temperature variation and humidity on the death of COVID-19 in Wuhan, China. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthi B. Resuscitation of microbial bioaerosols. In: Lighthart B., Mohr A.J., editors. Atmospheric Microbial Aerosols. Springer; Boston, MA: 1994. pp. 192–225. [DOI] [Google Scholar]
- Mayhall C.G. third ed. Lippincott Williams & Wilkins; Philadelphia: 2004. Hospital Epidemiology and Infection Control. [Google Scholar]
- McCluskey R., Sandin R., Greene J. Detection of airborne cytomegalovirus in hospital rooms of immuno-compromised patients. J. Virol. Methods. 1996;56:115–118. doi: 10.1016/0166-0934(95)01955-3. [DOI] [PubMed] [Google Scholar]
- McDevitt J.J., Rudnick S.N., Radonovich L.J. Aerosol susceptibility of influenza virus to UV-C light. Appl. Environ. Microbiol. 2012;78:1666–1669. doi: 10.1128/aem.06960-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney K.R., Gong Y.Y., Lewis T.G. Environmental transmission of SARS at Amoy Gardens. J. Environ. Health. 2006;68:26–30. [PubMed] [Google Scholar]
- Metz J.A., Finn A. Influenza and humidity – why a bit more damp may be good for you! J. Infect. 2015;71:S54–S58. doi: 10.1016/j.jinf.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Morawska L. Droplet fate in indoor environments, or can we prevent the spread of infection? Indoor Air. 2006;16:335–347. doi: 10.1111/j.1600-0668.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Morawska L., Cao J. Airborne transmission of SARS-CoV-2: the world should face the reality. Environ. Int. 2020;105730 doi: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K.L., Boehm A.B., Davies-Colley R.J., Dodd M.C., Kohn T., Linden K.G., Liu Y., Maraccini P.A., McNeill K., Mitch W.A., Nguyen T.H. Sunlight-mediated inactivation of health-relevant microorganisms in water: a review of mechanisms and modeling approaches. Environ. Sci. Process. Impacts. 2018;20:1089–1122. doi: 10.1039/C8EM00047F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J. Occup. Environ. Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermann F.J., Colfer R., Radzinski P., Robertson J. Proceedings of the 9th International Conference on Indoor Air Quality and Climate, Monterey, CA, June 30-July 5, 2002. 2002. Exposure to environmental tobacco smoke in an automobile; pp. 506–511. [Google Scholar]
- Oliveiros B., Caramelo L., Ferreira N.C., Caramelo F. Role of temperature and humidity in the modulation of the doubling time of COVID-19 cases. medRxiv. 2020 doi: 10.1101/2020.03.05.20031872. [DOI] [Google Scholar]
- Olsen S.J., Chang H.L., Cheung T.Y.Y., Tang A.F.Y., Fisk T.L., Ooi S.P.L., Kuo H.W., Jiang D.D.S., Chen K.T., Lando J., Hsu K.H., Chen T.J., Dowell S.F. Transmission of the severe acute respiratory syndrome on aircraft. N. Engl. J. Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott W., Klepeis N., Switzer P. Air change rates of motor vehicles and in-vehicle pollutant concentrations from secondhand smoke. J. Expo. Sci. Environ. Epidemiol. 2007;18:312–325. doi: 10.1038/sj.jes.7500601. [DOI] [PubMed] [Google Scholar]
- Papineni R.S., Rosenthal F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1997;10:105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- Park J., Spengler J.D., Yoon D., Dumyahn T., Lee K., O zkayak H. Measurement of air exchange rate of stationary vehicles and estimation of in- vehicle exposure. J. Expo. Anal. Environ. Epidemiol. 1998;8:65–78. [PubMed] [Google Scholar]
- Park J.E., Son W.S., Ryu Y., Choi S.B., Kwon O., Ahn I. Effects of temperature, humidity, and diurnal temperature range on influenza incidence in a temperate region. Influenza Other Resp. 2019;14:11–18. doi: 10.1111/irv.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier C., Luo W., Majumder M.S., Liu D., Mandl K., Mooring T., Santillana M. 2020. The Role of Environmental Factors on Transmission Rates of the COVID-19 Outbreak: an Initial Assessment in Two Spatial Scales. Available at SSRN 3552677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Z., Ye Y., Chang P.H., Thirunarayanan D., Wigginton K.R. Nucleic acid photolysis by UV254 and the impact of virus encapsidation. Environ. Sci. Technol. 2018;52:10408–10415. doi: 10.1021/acs.est.8b02308. [DOI] [PubMed] [Google Scholar]
- Radonovich L.J., Simberkoff M.S., Bessesen M.T., Brown A.C., Cummings D.A., Gaydos C.A., Los J.G., Krosche A.E., Gibert C.L., Gorse G.J., Nyquist A.C. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. Jama. 2019;322:824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrow J., Mao S., Celik I., Posada J.A., Feng Z. Modeling the evaporation and dispersion of airborne sputum droplets expelled from a human cough. Build. Environ. 2011;46:2042–2051. doi: 10.1016/j.buildenv.2011.04.011. [DOI] [Google Scholar]
- Rzeżutka A., Cook N. Survival of human enteric viruses in the environment and food. FEMS Microbiol. Rev. 2004;28:441–453. doi: 10.1016/j.femsre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Sandaradura I., Goeman E., Pontivivo G., Fine E., Gray H., Kerr S., Marriott D., Harkness J., Andresen D. A close shave? Performance of P2/N95 respirators in health care workers with facial hair: results of the BEARDS (Adequate Respiratory DefenceS) study. J. Hosp. Infect. 2020;104:529–533. doi: 10.1016/j.jhin.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Schuit M., Gardner S., Wood S., Bower K., Williams G., Freeburger D., Dabisch P. The influence of simulated sunlight on the inactivation of influenza virus in aerosols. J. Infect. Dis. 2020;221:372–378. doi: 10.1093/infdis/jiz582. [DOI] [PubMed] [Google Scholar]
- Science Media Center (SMC) 2020. Expert Reaction to Questions about COVID-19 and Viral Load.https://www.sciencemediacentre.org/expert-reaction-to-questions-about-covid-19-and-viral-load/ [Google Scholar]
- Sean W.X.O., Yian K.T., Po Y.C., Tau H.L., Oon T.N., Michelle S.Y.W., Kalisvar M. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. Jama. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Kohn M. Absolute humidity modulates influenza survival, transmission, and seasonality. Proc. Natl. Acad. Sci. 2009;106:3243–3248. doi: 10.1073/pnas.0806852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu E.Y.C., Leung N.H.L., Cowling B.J. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr. Opin. Infect. Dis. 2019;32:372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- Siegel J.D., Rhinehart E., Jackson M., Chiarello L., Strausbaugh L. Centers for Disease Control and Prevention (CDC); 2007. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings.https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D., Gendreau M. Medical issues associated with commercial flights. Lancet. 2009;373:2067–2077. doi: 10.1016/S0140-6736(09)60209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H.H., Bradish C.J. Exposure to light as a source of error in the estimation of the infectivity of virus suspensions. Microbiology. 1954;10:377–397. doi: 10.1099/00221287-10-3-377. [DOI] [PubMed] [Google Scholar]
- Stanam A., Chaudhari M., Rayudu D. Effects of temperature on COVID-19 transmission. medRxiv. 2020 doi: 10.1101/2020.03.29.20044461. [DOI] [Google Scholar]
- Tan J., Mu L., Huang J., Yu S., Chen B., Yin J. An initial investigation of the association between the SARS outbreak and weather: with the view of the environmental temperature and its variation. J. Epidemiol. Community Health. 2005;59:186–192. doi: 10.1136/jech.2004.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface. 2009;6:S737–S746. doi: 10.1098/rsif.2009.0227.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.W., Lai F.Y.L., Wong F., Hon K.L.E. Incidence of common respiratory viral infections related to climate factors in hospitalized children in Hong Kong. Epidemiol. Infect. 2010;138:226–235. doi: 10.1017/S0950268809990410. [DOI] [PubMed] [Google Scholar]
- Tang J.W., Li Y., Eames I., Chan P.K.S., Ridgway G.L. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J. Hosp. Infect. 2006;64:100–114. doi: 10.1016/j.jhin.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. Review of aerosol transmission of influenza A virus. Emerg. Infect. Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R. Aerosol transmission of influenza A virus: a review of new studies. J. R. Soc. Interface. 2009;6:S783–S790. doi: 10.1098/rsif.2009.0302.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect. Dis. 2019;19 doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The American Institute of Architects Academy of Architecture for Health (AIA) 2001. Guidelines for Design and Construction of Hospitals and Health Care Facilities. Washington.https://www.brikbase.org/content/guidelines-design-and-construction-hospitals-and-healthcare-facilities [Google Scholar]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liuxingbingxue Zazhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R.J. Particle size and pathogenicity in the respiratory tract. Virulence. 2013;4:847–858. doi: 10.4161/viru.27172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosepu R., Gunawan J., Effendy D.S., Lestari H., Bahar H., Asfian P. Correlation between weather and Covid-19 pandemic in Jakarta, Indonesia. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.138436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng C.C., Li C.S. Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J. Occup. Environ. Hyg. 2007;4:400–405. doi: 10.1080/15459620701329012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sande M., Teunis P., Sabel R. Professional and home-made face masks reduce exposure to respiratory infections among the general population. PloS One. 2008;3 doi: 10.1371/journal.pone.0002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Bushmaker T., Munster V.J. Stability of Middle East respiratory syndrome coronavirus (MERS-CoV) under different environmental conditions. Euro Surveill. 2013;18:20590. doi: 10.2807/1560-7917.ES2013.18.38.20590. [DOI] [PubMed] [Google Scholar]
- Viboud C., Alonso W.J., Simonsen L. Influenza in tropical regions. PLoS Med. 2006;3:e89. doi: 10.1371/journal.pmed.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Du G. COVID-19 may transmit through aerosol. Ir. J. Med. Sci. 2020:1–2. doi: 10.1007/s11845-020-02218-2. (1971–) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Tang K., Feng K., Lv W. 2020. High Temperature and High Humidity Reduce the Transmission of COVID-19. Available at SSRN 3551767. [DOI] [Google Scholar]
- Wang M., Jiang A., Gong L., Luo L., Guo W., Li C., Zheng J., Li C., Yang B., Zeng J., Chen Y. Temperature significant change COVID-19 Transmission in 429 cities. MedRxiv. 2020 doi: 10.1101/2020.02.22.20025791. [DOI] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells W.F. On air-borne infection: study II. Droplets and droplet nuclei. Am. J. Epidemiol. 1934;20:611–618. doi: 10.1093/oxfordjournals.aje.a118097. [DOI] [Google Scholar]
- Wenzel R.P. fourth ed. Lippincott Williams & Wilkins; Philadelphia: 2003. Prevention and Control of Nosocomial Infections. [Google Scholar]
- Wenzel R.P., Edmond M.B. Managing SARS amidst uncertainty. N. Engl. J. Med. 2003;348:1947–1948. doi: 10.1056/NEJMp030072. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2004. Technical Report, Series No. 924.https://www.who.int/biologicals/technical_report_series/en/ [PubMed] [Google Scholar]
- World Health Organization (WHO) 2007. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Diseases in Health Care — WHO Interim Guidelines.https://www.who.int/csr/bioriskreduction/infection_control/publication/en/ (Updated: April 2014) [PubMed] [Google Scholar]
- World Health Organization (WHO) 2009. Natural Ventilation for Infection Control in Health-Care Settings.https://www.who.int/water_sanitation_health/publications/natural_ventilation/en/ [PubMed] [Google Scholar]
- World Health Organization (WHO) 2014. Infection Prevention and Control of Epidemic and Pandemic Prone Acute Respiratory Infections in Healthcare – WHO Guidelines.https://www.who.int/csr/bioriskreduction/infection_control/publication/en/ [PubMed] [Google Scholar]
- World Health Organization (WHO) 2020. Coronavirus Disease (COVID-2019) Situation Reports, 05 May 2020.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ [Google Scholar]
- World Health Organization (WHO) 2020. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations: Scientific Brief, 27 March 2020.https://apps.who.int/iris/handle/10665/331601 [Google Scholar]
- World Health Organization (WHO) 2020. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) 16-24 February 2020.https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf [Google Scholar]
- World Health Organization (WHO) 2020. Infection Prevention and Control Guidance for COVID-19.https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 [Google Scholar]
- World Health Organization (WHO) 2020. Management of Ill Travellers at Points of Entry—International Airports, Seaports and Ground Crossings—In the Context of COVID-19 Outbreak.https://www.who.int/publications-detail/management-of-ill-travellers-at-points-of-entryinternational-airports-seaports-and-groundcrossings-in-the-context-of-covid-19-outbreak [Google Scholar]
- Wu Y., Jing W., Liu J., Ma Q., Yuan J., Wang Y., Du M., Liu M. Effects of temperature and humidity on the daily new cases and new deaths of COVID-19 in 166 countries. Sci. Total Environ. 2020:139051. doi: 10.1016/j.scitotenv.2020.139051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Li Y., Chwang A.T., Ho P.L., Seto W.H. How far droplets can move in indoor environments--revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Yang L., Li X., Yan Y., Tu J. Effects of cough-jet on airflow and contaminant transport in an airliner cabin section. J. Comput. Multiph. Flows. 2017;10:72–82. doi: 10.1177/1757482X17746920. [DOI] [Google Scholar]
- Yang W., Elankumaran S., Marr L.C. Relationship between humidity and influenza A viability in droplets and implications for influenza's seasonality. PloS One. 2012;7 doi: 10.1371/journal.pone.0046789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J. Med. Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiqing L., Yongyun C., Wenxiang C., Mengning Y., Yuanqing M., Zhenan Z., Haishan W., Jie Z., Kerong D., Huiwu L., Fengxiang L. Surgical masks as source of bacterial contamination during operative procedures. J. Orthop. Transl. 2018;14:57–62. doi: 10.1016/j.jot.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J. Association between ambient temperature and COVID-19 infection in 122 cities from China. Sci. Total Environ. 2020;724 doi: 10.1016/j.scitotenv.2020.138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]