Table 1.
Reaction Scopea
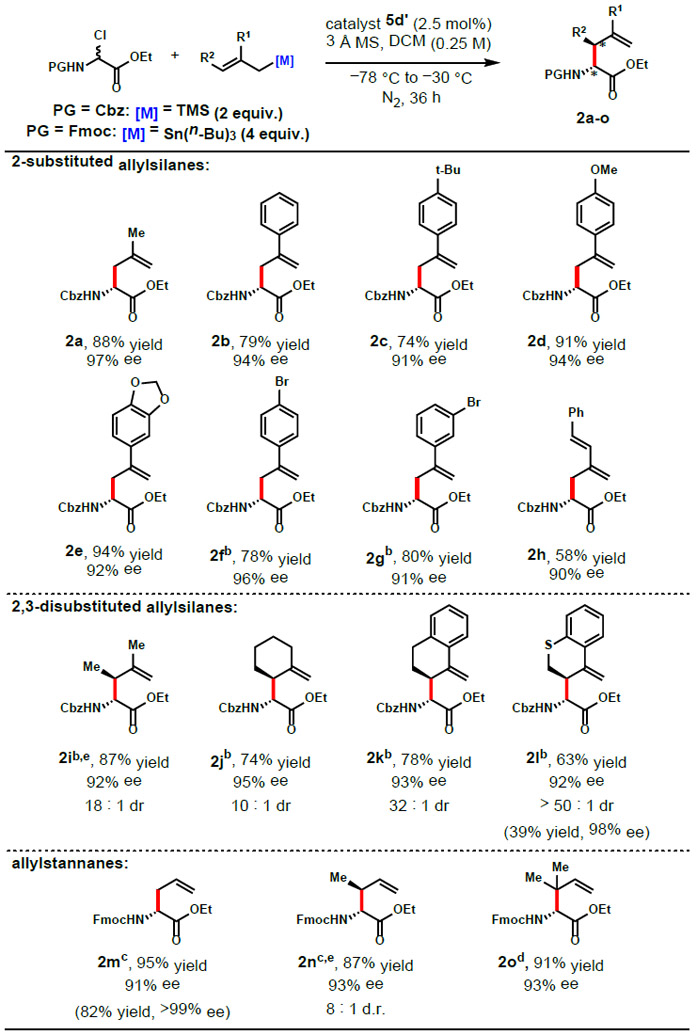 |
Conditions: substrate (0.5 mmol), catalyst (0.0125 mmol), nucleophile (1.0 mmol), 3 Å MS (60 mg), DCM (2 mL), under N2, initially cooled to −78 °C and stirred at −30 °C, 36 h. Enantiomeric excess determined by HPLC. Diastereomeric ratio determined by 1H NMR of the crude product, yield reflects isolation of major diasteromer.
−5 °C.
catalyst (0.05 mmol), nucleophile (2.0 mmol), DCM (5 mL), −50 °C, 72 h.
catalyst (0.05 mmol), nucleophile (1.5 mmol), DCM (5 mL), −30 °C, 72 h.
(E)-trimethyl(2-methylbut-2-en-1-yl)silane and (E)-crotylstannane employed as the nucleophiles. Yields and enantioselectivities in parentheses are of crystallized products.
