FIGURE 2.
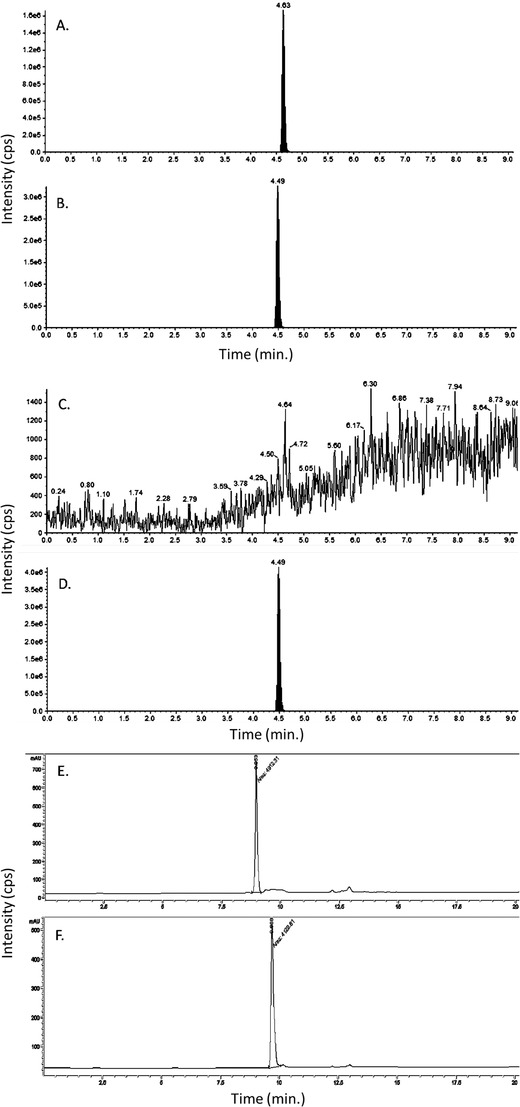
Validation of the active pharmaceutical ingredient of verubecestat. Chromatogram of standard verubecestat (A), catalog S8564, and MK‐2206 (B) the internal standard injected to liquid chromatography‐mass spectrometry (LC/MS/MS) system. The filled peak is the analyte of interest. Chromatogram of custom batch active pharmaceutical ingredient (API) verubecestat (C), catalog S8564, and MK‐2206 (D) the internal standard injected to LC/MS/MS system. The filled peak is the analyte of interest. Chromatogram of standard verubecestat (E) at retention time 8.953 minutes, and custom batch API verubecestat (F) at retention time 9.666 minutes determined not to be the correct API for verubecestat, injected to liquid chromatography/ultraviolet (LC/UV) system
