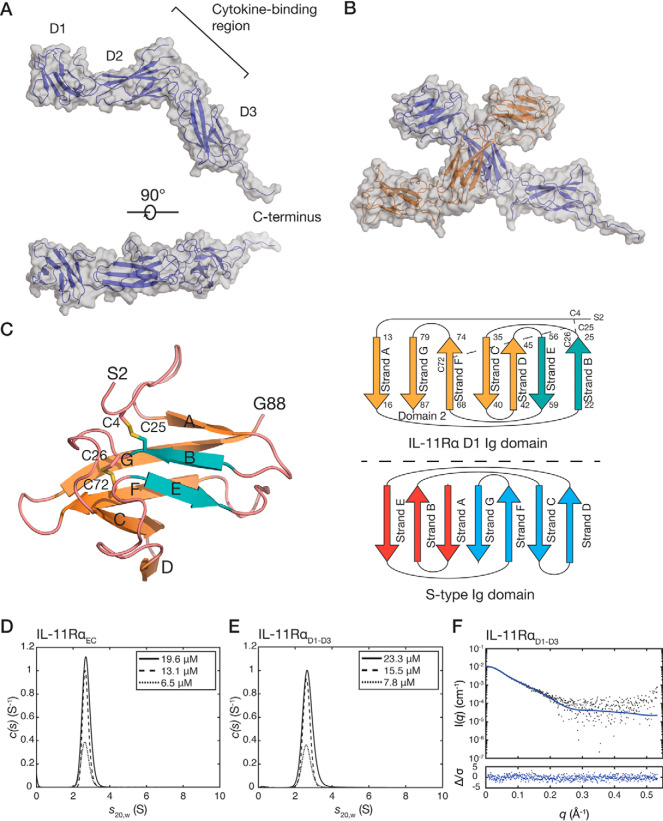
Figure 1.
The crystal structure of IL-11RαEC. A, two views of the structure of IL-11RαEC. Each of the domains and the section of the C-terminus that is defined in the electron density are indicated. The transmembrane domain is at the C-terminal region of the receptor. B, the asymmetric unit of the IL-11Rα crystal structure, formed by two IL-11Rα molecules, with an extensive contact between D2 of the two molecules. C, the structure (left panel) and topology (top right panel) of D1 from chain B of IL-11RαEC with disulfide bonds indicated. Loops are colored pink, the two strands contributing to the smaller, anti-parallel β-sheet are blue, and the five strands contributing to the larger, mixed parallel/anti-parallel β-sheet are orange. A topology diagram of the typical s-type Ig domain is also shown (bottom right panel). D, continuous sedimentation coefficient (c(s)) distributions for IL-11RαEC at three concentrations, showing that IL-11RαEC is primarily monomeric in solution under the conditions tested. Slight concentration dependence in the sedimentation coefficient suggests the formation of a transient oligomer. E, c(s) distributions for IL-11RαD1–D3 at several concentrations. F, small-angle X-ray scattering data for IL-11RαD1–D3, overlaid with the theoretical scattering profile calculated from molecule A of the crystal structure of IL-11RαEC (χ2 = 1.05).