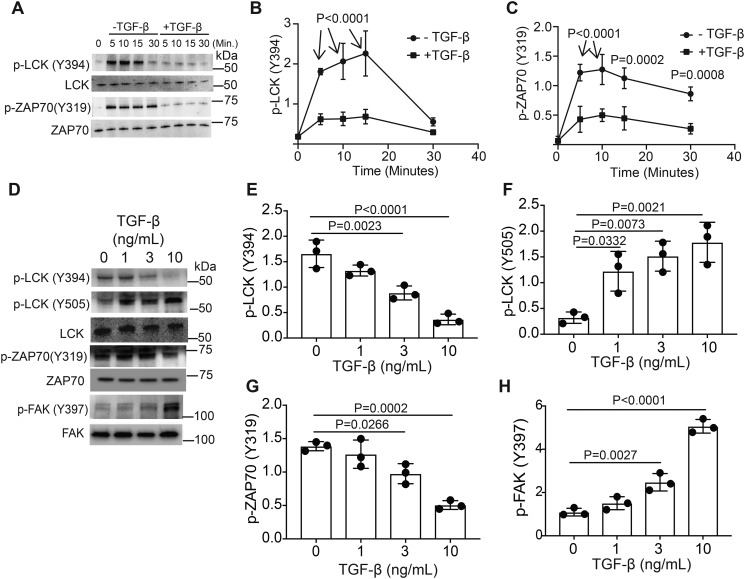
Figure 1.
TGF-β signaling alters LCK and ZAP70 activation in murine CD4+ T cells. Murine CD4+ T cells isolated by negative selection were in vitro activated through TCR and CD28 costimulation for various time points with or without 10 ng/ml TGF-β. A, immunoblotting was performed on cell lysates for the phosphorylated and total protein LCK and ZAP-70 abundance. B and C, densitometry was performed across three independent experiments to quantitate the abundance of tyrosine phosphorylation for (B) LCK (Tyr-394) and (C) ZAP70 (Tyr-319). The abundance of phosphorylated protein was normalized to total protein. Shown are mean ± S.D.; p values were calculated by two-way ANOVA. Murine CD4+ T cells isolated by negative selection were in vitro activated through TCR and CD28 costimulation for 10 min with various doses of TGF-β. D, immunoblotting was performed on cell lysates for the phosphorylated and total protein abundance for LCK, ZAP-70, and FAK. E–H, densitometry was performed across three independent experiments to quantitate the abundance of tyrosine phosphorylation for (E) LCK (Tyr-394), (F) LCK (Tyr-505), (G) ZAP70 (Tyr-319), and (H) FAK (Tyr-397). The abundance of phosphorylated protein was normalized to total protein. Shown are mean ± S.D.; p values were calculated by one-way ANOVA with a Tukey multiple comparison test.