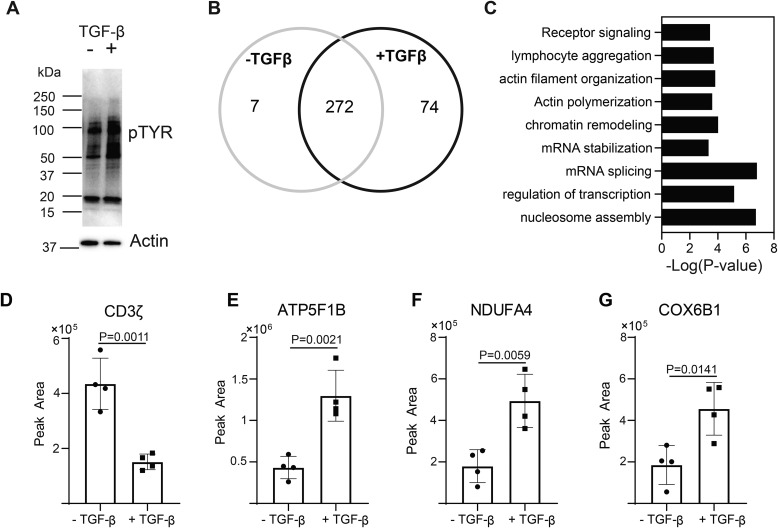
Figure 2.
TGF-β signaling alters phospho-tyrosine signaling networks in CD4+ T cells. Murine CD4+ T cells isolated by negative selection were in vitro activated through TCR and CD28 stimulation for 10 min ± 10 ng/ml TGF-β. A, immunoblotting was performed on cell lysates using the anti–phospho-tyrosine antibody. One representative blot from three independent experiments is shown. Tyrosine phosphorylated proteins were immunoprecipitated from T cells activated for 10 min ± 10 ng/ml TGF-β. Label-free quantitative MS was used to identify proteins in the phospho-tyrosine IPs. B, a Venn diagram was constructed using a 2-fold cutoff based on the label-free quantitative data to illustrate the number of tyrosine-phosphorylated proteins enriched in T cells activated in the presence or absence of TGF-β from four or five independent experiments. C, the distribution of tyrosine-phosphorylated proteins specific to T cells activated through TCR-CD28 plus TGF-β were compared with the mouse proteome with a binomial test for gene ontology and biological pathway in the PANTHER software package. The probability (p value) that the number of proteins in each group occurred by chance was calculated. D–G, representative examples of the label-free quantitation of the mass spectrometric data are illustrated for (D) CD3ζ, (E) ATP5F1B, (F) NDUFA4, and (G) COX5B1. Shown are mean ± S.D.; p values were calculated with a two-tailed Student's t test.