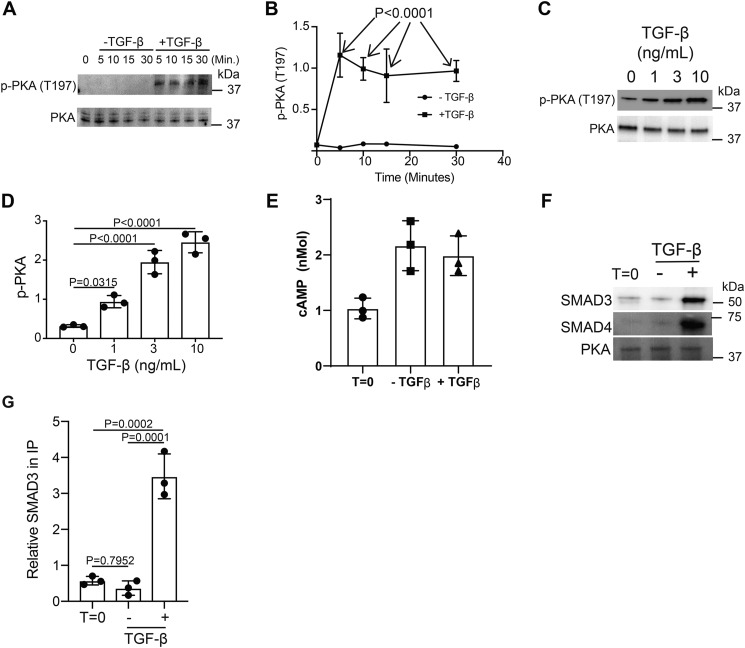
Figure 5.
TGF-β signaling activates PKA via SMAD3/4 binding. Murine CD4+ T cells isolated by negative selection were in vitro activated through TCR and CD28 costimulation for various time points with or without 10 ng/ml TGF-β. A, immunoblotting was performed on cell lysates for the phosphorylated and total protein abundance for PKA. B, densitometry was performed across three independent experiments to quantitate the abundance of tyrosine phosphorylation for PKA (Thr-197). The abundance of phosphorylated protein was normalized to total protein. Shown are mean ± S.D.; p values were calculated by two-way ANOVA. T cells isolated by negative selection were in vitro activated through TCR and CD28 costimulation for 10 min with various amounts of TGF-β. C, immunoblotting was performed for p-PKA (Thr-197) and total PKA. D, densitometry across three independent immunoblots was performed for p-PKA (Thr-197) normalized to total PKA. Shown are mean ± S.D.; p values were calculated by one-way ANOVA with a Tukey multiple comparison test. E, cAMP levels were measured in resting (T = 0) or T cells activated for 10 min in the presence or absence of 10 ng/ml TGF-β (n = 3). CD4+ T cells isolated by negative selection were in vitro activated through TCR and CD28 costimulation for 10 min ± 10 ng/ml TGF-β. F, PKA was immunoprecipitated and immunoblotted for SMAD3, SMAD4, and PKA. One representative blot from three independent experiments is shown. G, densitometry across three independent immunoblots was performed for SMAD3 and normalized to total PKA in the IP. Shown are mean ± S.D.; p values were calculated by one-way ANOVA with a Tukey multiple comparison test.