Figure 1.
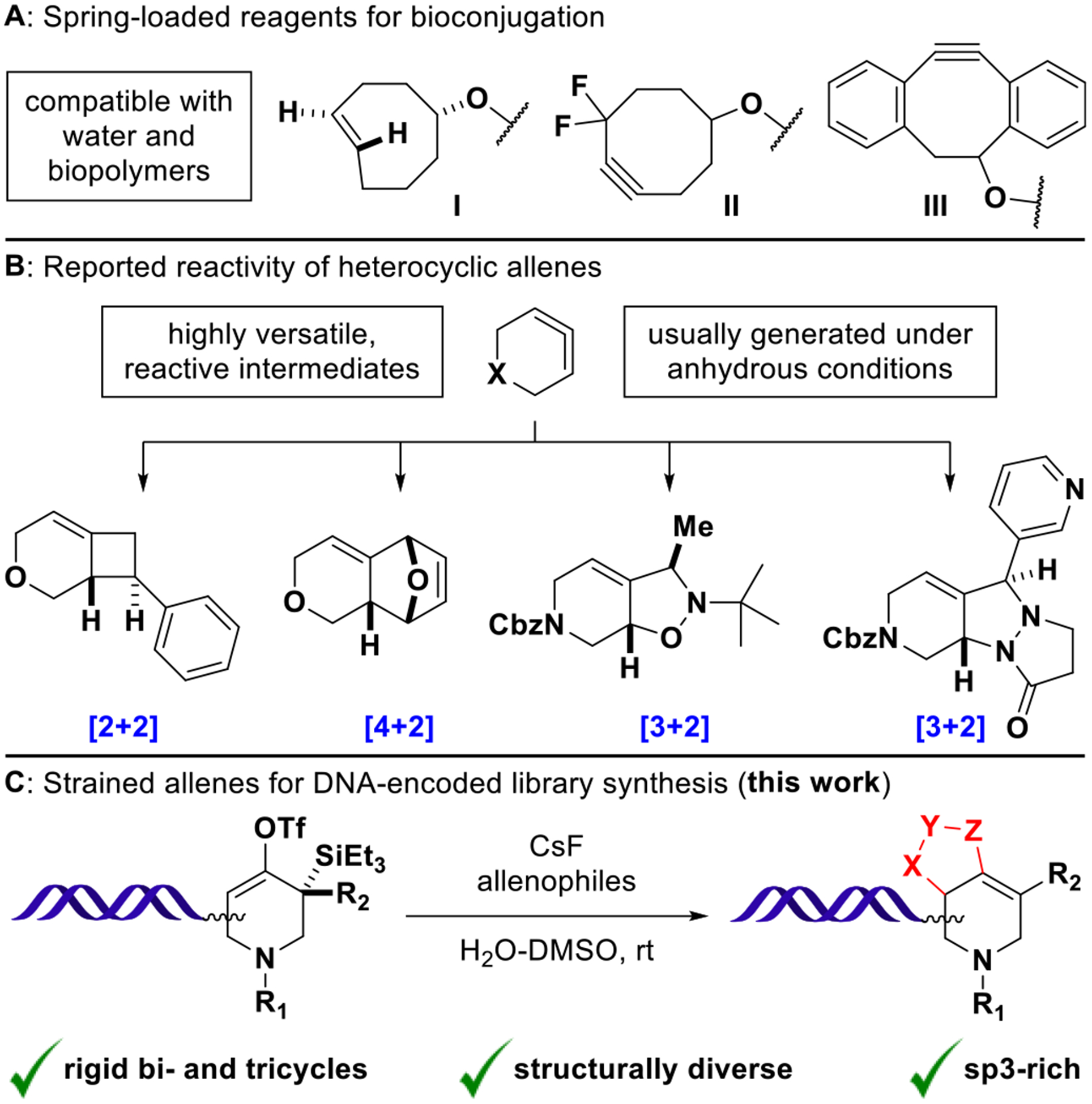
(A) Water-compatible reagents for bioorthogonal chemistry: trans-Cyclooctene (I) rapidly undergoes inverse-electron-demand Diels–Alder reactions with tetrazines. Cyclooctyne derivatives (II, III) undergo strain-promoted azide–alkyne cycloadditions. (B) In situ generated heterocyclic allenes exhibit distinct cycloaddition modes with activated olefins, dienes, and 1,3-dipoles (major diastereomers shown). (C) Fluoride-induced formation of DNA- conjugated heterocyclic allenes and trapping with various allenophiles affords structurally diverse cycloaddition products.
