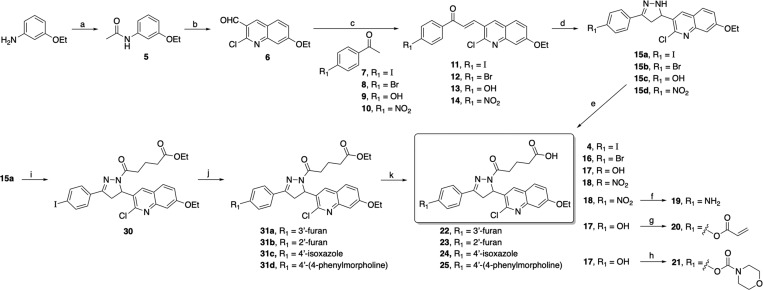
Scheme 1. Synthesis of Compound 4 and Analogs 16–25.
Reagents and conditions: (a) acetic anhydride, DIPEA, DMAP, DCM, rt for 2 h, 88%; (b) (i) DMF, POCl3, 0 °C for 25 min, (ii) acetanilide 5, 110 °C for 3 h, 74%; (c) 2.5 M NaOH, EtOH, 45 °C for 45 min to 1 h, 53–74%; (d) hydrazine hydrate, EtOH, reflux for 2–3 h, 70–83%; (e) glutaric anhydride, CHCl3, reflux for 2 h, 58–71% (after recrystallization); (f) SnCl2, EtOH:THF (1:1), reflux for 2 h, 48% (after recrystallization); (g) acryloyl chloride, 2 N NaOH, THF, 0 °C to rt for 2 h, 34%; (h) 4-morpholinecarbonyl chloride, TEA, DMAP, THF, 0 °C to rt for 12 h, 63%. (i) ethyl glutaryl chloride, DIPEA, DCM, rt for 12 h, 78%; (j) boronic acid/ester, Pd(PPh3)4, CsF, DME, 90 °C for 15–18 h, 63–69%; (k) 10 N NaOH, THF:MeOH (1:2), rt for 6–8 h, 75–87%.