Fig. 2.
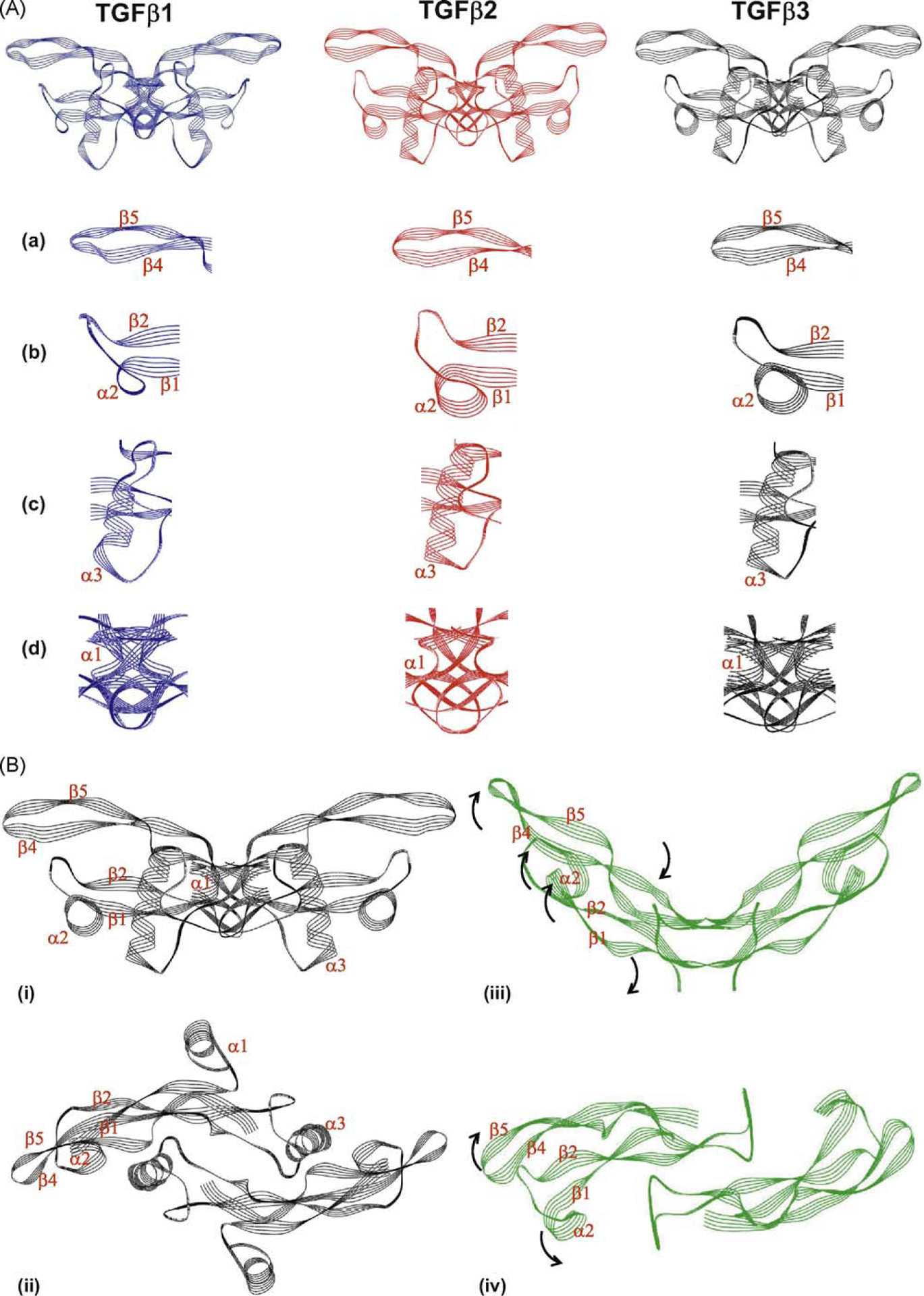
The structure of TGF-β3 differs from that of the other TGF-β isoforms and is capable of undergoing extensive rearrangement. (A) The structures of TGF-β1 (blue), TGF-β2 (red) and TGF-β3 (black) in their ‘closed’ form are shown (the dimeric forms of each isoform consisting of two monomers are represented). Domains of the protein which differ with the other isoforms are highlighted in the panels beneath each isoform; (a) β-strand β4 and β5; (b) α-helix α2; (c) a-helix α3 (d) monomer interface comprising N-terminal, a-helix a1 and the C-terminal domain. (B) Structural differences between ‘closed’ and ‘open’ forms of TGF-β3. The ‘closed’ structure of TGF-β3 is shown in black in two orientations (i) side-on and (ii) from above; while the ‘open’ structure of TGF-β3 is represented in green in two orientations (iii) side-on and (iv) from above. Individual peptide motifs of the TGF-β3 protein that undergo steric rearrangement are denoted on the individual representations. The direction of the steric rearrangement of peptide motifs between the ‘closed and ‘open’ forms is denoted by black arrows on the open form. Note that in the ‘open’ form the structure of the α-helices α1 and α3 is not represented as these motifs are disordered and therefore co-ordinates are not available. The structures of the individual isoforms are derived from co-ordinates previously published [12,17,146–148]. Images were generated using Weblab Viewer Lite (Molecular Simulations Inc. (now Accelrys)); superpositions of the structures were performed with a previously written software [149]. The structural alignments were based on the carbon a-atoms of the similar residues (as indicated by the sequential alignments) of the individual TGF-β isoforms. Other proteins that were reported in the published structures were disregarded for this analysis.
