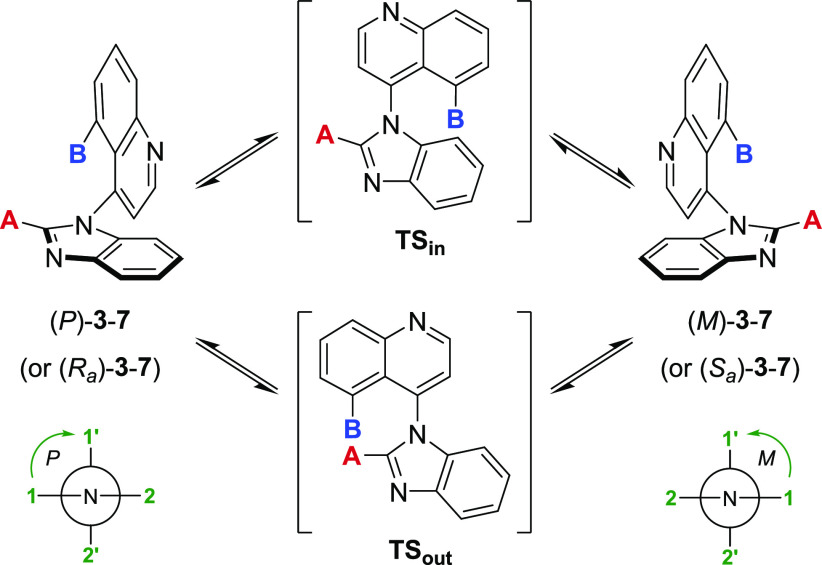
Table 1. Rotational Barriers of Related 4-(1H-Benzo[d]imidazol-1-yl)quinolines.
| Cpd | A | B | ΔErot (TSin) (kcal/mol)a | ΔErot (TSout) (kcal/mol)a | Atropisomer classb |
|---|---|---|---|---|---|
| 3 | H | H | 29.6 | 14.1 | 1 |
| 4 | H | F | 33.7 | 15.5 | 1 |
| 5 | H | CH3 | >40 | 22.5 | 2 |
| 6 | CH3 | H | 32.2 | 28.3 | 2 |
| 7 | CH3 | F | 37.7 | 33.9 | 3 |
Using MMFFs force field, the best estimates for the rotational barriers ΔErot via TSin and TSout were obtained from the lowest energy pathway in either direction (clockwise or counterclockwise).19
Class 1: ΔErot < 20 kcal/mol; Class 2: 20 kcal/mol < ΔErot < 30 kcal/mol; Class 3: ΔErot > 30 kcal/mol.