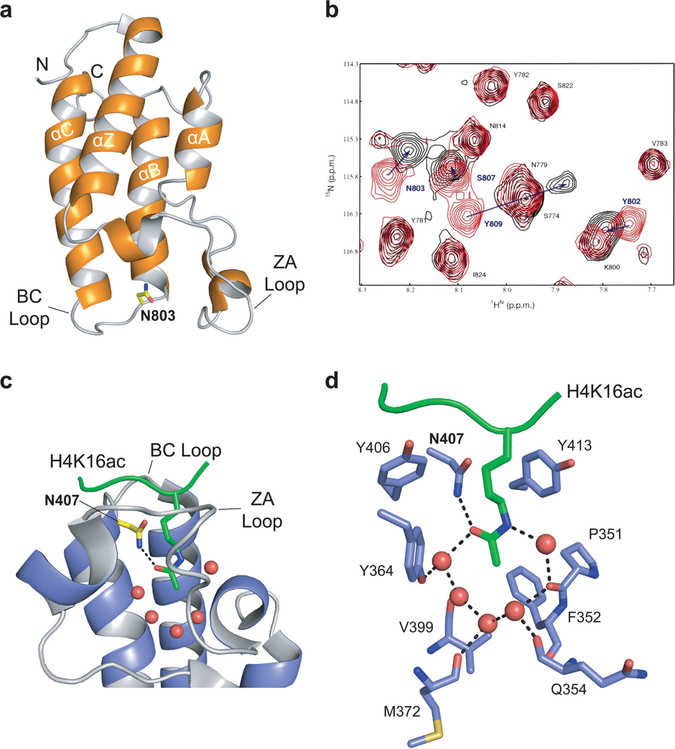
Figure 1.
Bromodomain as the acetyl-lysine reader domain. (a) NMR structure of the PCAF bromodomain (PDB: 1N72). The four helices (αZ, αA, αB, αC) are noted, as are the N and C termini of the structure and the ZA and BC loops. The conserved asparagine residue, N803, is highlighted in yellow. (b) 2D 1H–15N-heteronuclear single quantum coherence (HSQC) spectra of the PCAF bromodomain (~0.5 mM) in its free form (red) and in complex with the acetylated H4 peptide (molar ratio 1:6; black). Figure adapted with permission from ref 17. Copyright 1999 Macmillan Publishers Limited. (c) Crystal structure of the GCN5 bromodomain (blue) in complex with the acetylated peptide H4K16ac (green; PDB: 1E6I). The five water molecules at the base of the bromodomain binding pocket are represented as red spheres, the conserved asparagine residue, N407, is highlighted in yellow, and the ZA and BC loops are labeled. The key hydrogen bond between the conserved asparagine residue and the acetyl-lysine is represented by a black dashed line. (d) Stick diagram of the network of interactions among the H4K16ac peptide and the residues and water molecules within the GCN5 binding pocket. Key residues are labeled. Water molecules are represented by red spheres, and the hydrogen bonding network is represented by black dashed lines.