Abstract
Oligodendrocyte lineage cells (oligodendroglia) and neurons engage in bidirectional communication throughout life to support healthy brain function. Recent work shows that changes in neuronal activity can modulate proliferation, differentiation, and myelination to support the formation and function of neural circuits. While oligodendroglia express a diverse collection of receptors for growth factors, signaling molecules, neurotransmitters and neuromodulators, our knowledge of the intracellular signaling pathways that are regulated by neuronal activity remains largely incomplete. Many of the pathways that modulate oligodendroglia behavior are driven by changes in intracellular calcium signaling, which may differentially affect cytoskeletal dynamics, gene expression, maturation, integration, and axonal support. Additionally, activity-dependent neuron-oligodendroglia communication plays an integral role in the recovery from demyelinating injuries. In this review, we summarize the modalities of communication between neurons and oligodendroglia and explore possible roles of activity-dependent calcium signaling in mediating cellular behavior and myelination.
Highlights
• Neuron-oligodendroglia signaling is essential for myelination and circuit function
• Neuronal activity modulates intracellular calcium in oligodendroglia
• Intracellular calcium affects cytoskeleton, gene expression, and metabolic support
• Neuronal activity-dependent signaling drives remyelination and disease recovery
Introduction
Myelination by oligodendrocytes increases conduction velocity, metabolic efficiency, energetic support, and information processing capacity of the central nervous system (CNS, [1]). Oligodendrocytes are generated through the differentiation of oligodendrocyte precursor cells (OPCs, Fig. 1). During development, these proliferative precursors migrate from germinal zones to distribute throughout the CNS. OPCs persist in the adult nervous system in an evenly-spaced, tiled distribution throughout life, where they retain the potential to differentiate into new oligodendrocytes. Dynamic, bidirectional communication between neurons and oligodendrocyte lineage cells regulates multiple stages of cellular maturation, including OPC differentiation and mature oligodendrocyte integration into neuronal circuits (Fig. 1). Since Barres and Raff [2] and Demerens et al. [3] demonstrated that electrical activity modulates oligodendrocyte precursor proliferation and myelination in the optic nerve, considerable progress has been made in understanding the complex relationship between active axons and oligodendrocyte lineage cells throughout life.
Figure 1 |. Oligodendroglia associate with axons throughout maturation.
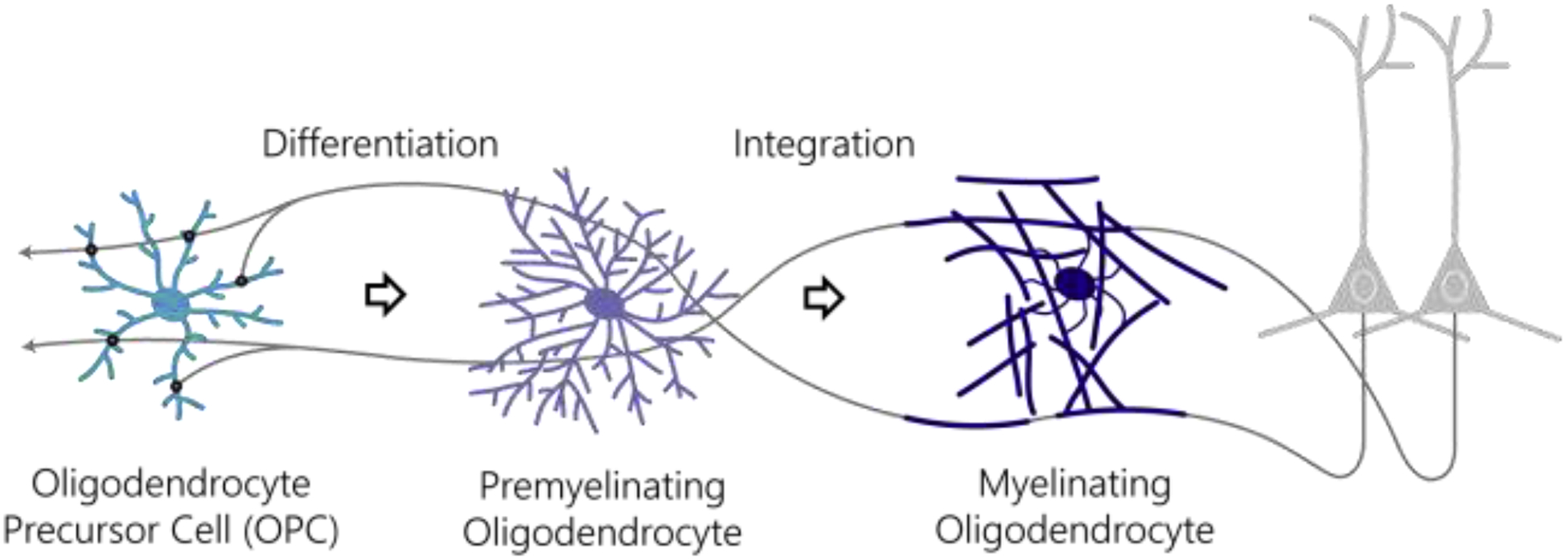
OPCs (light blue) receive excitatory and inhibitory synaptic input from neurons (grey). Neuronal activity-dependent signals drive the differentiation of OPCs into premyelinating oligodendrocytes (purple) and the subsequent integration of mature oligodendrocytes (dark blue) which myelinate neuronal circuits.
Reciprocal signaling between active neurons and oligodendroglia has proven essential to shape patterns of myelination and tune circuit function. During development, seminal studies in the zebrafish showed that activity-dependent neuronal vesicular release is a primary driver of axon selection and myelin ensheathment during development [4,5]. In the adult brain, additional evidence for activity-dependent regulation of oligodendrogenesis and mature oligodendrocyte integration into circuits has been revealed using electrical [6], optogenetic [7], pharmacogenetic [8], and sensory stimulation techniques [9] in the mouse. Furthermore, eliminating the generation of new oligodendrocytes in the adult nervous system decreased the ability of mice to learn and retrieve new memories, suggesting an exciting role for myelination in the formation and consolidation of novel neural circuits [10–13]. These and other studies have provided a foundation for the field moving forward, as we continue to dissect the dynamic interplay between neuronal activity, oligodendroglial cell signaling, and brain plasticity.
These findings are mirrored in humans, where new oligodendrocytes are generated continuously in the cerebral cortex and life experience may modulate the myelination of axons [14]. Juggling and practicing the piano increased white matter microstructure in related brain regions [15,16], while social isolation in children leads to decreased development of white matter tracts connected to the prefrontal cortex [17,18]. Taken together, these data demonstrate that neuronal activity regulates the dynamics of oligodendrocyte lineage cells to facilitate healthy brain function, learning, and behavior. However, the precise mechanisms through which neuronal activity-dependent signals are transduced into complex intracellular changes within the oligodendrocyte lineage remain unclear. In this review, we will focus on recent advances that have increased our understanding of the extrinsic and intracellular signaling mechanisms that allow oligodendrocyte lineage cells to sense and respond to neuronal activity and support neural plasticity.
1. Detection of neuronal activity by the oligodendrocyte lineage
Neuron-OPC synapses
While it is clear that neuronal activity modulates cells of the oligodendrocyte lineage, the biological mechanisms underlying this interaction remain ill defined. Pioneering work by Bergles et al. [19] demonstrated that neurons make functional excitatory synapses on OPC processes using whole cell patch clamp recording in hippocampal slices. In these experiments, Schaffer collateral stimulation elicited fast, quantal, excitatory postsynaptic potentials in CA1 OPCs that were mediated by α-amino-3-hydroxy-5-methyl isoxazolepropionic acid (AMPA) receptors and exhibited paired-pulse facilitation similar to that of pyramidal neurons. Electron microscopy revealed direct synaptic junctions on OPC processes with clustered presynaptic vesicles and postsynaptic membrane densities. Subsequent research has defined the electrophysiological properties of OPCs and continues to work toward an understanding of the functional relevance of this unique cellular interaction.
OPCs are specialized to detect neuronal activity through the expression of numerous neurotransmitter receptors and ion channels (Fig. 2, [20]; reviewed in Larson et al., [21]). While OPCs have been shown to receive synaptic input in all brain regions explored [19,22–24], there is region- and age-specific heterogeneity in receptor and ion channel expression [24–26] suggesting that the microenvironment may regulate their cellular behavior. Recently, monosynaptic viral tracing of OPC afferents indicated that OPCs of the cortex and corpus callosum are connected to widely distributed neuronal networks throughout the brain [27]. How OPCs integrate and respond to synaptic information from diverse brain regions and cell types remains an open question and an active area of investigation in the field.
Figure 2 |. Neuronal activity-dependent calcium signaling pathways that regulate OPC differentiation.
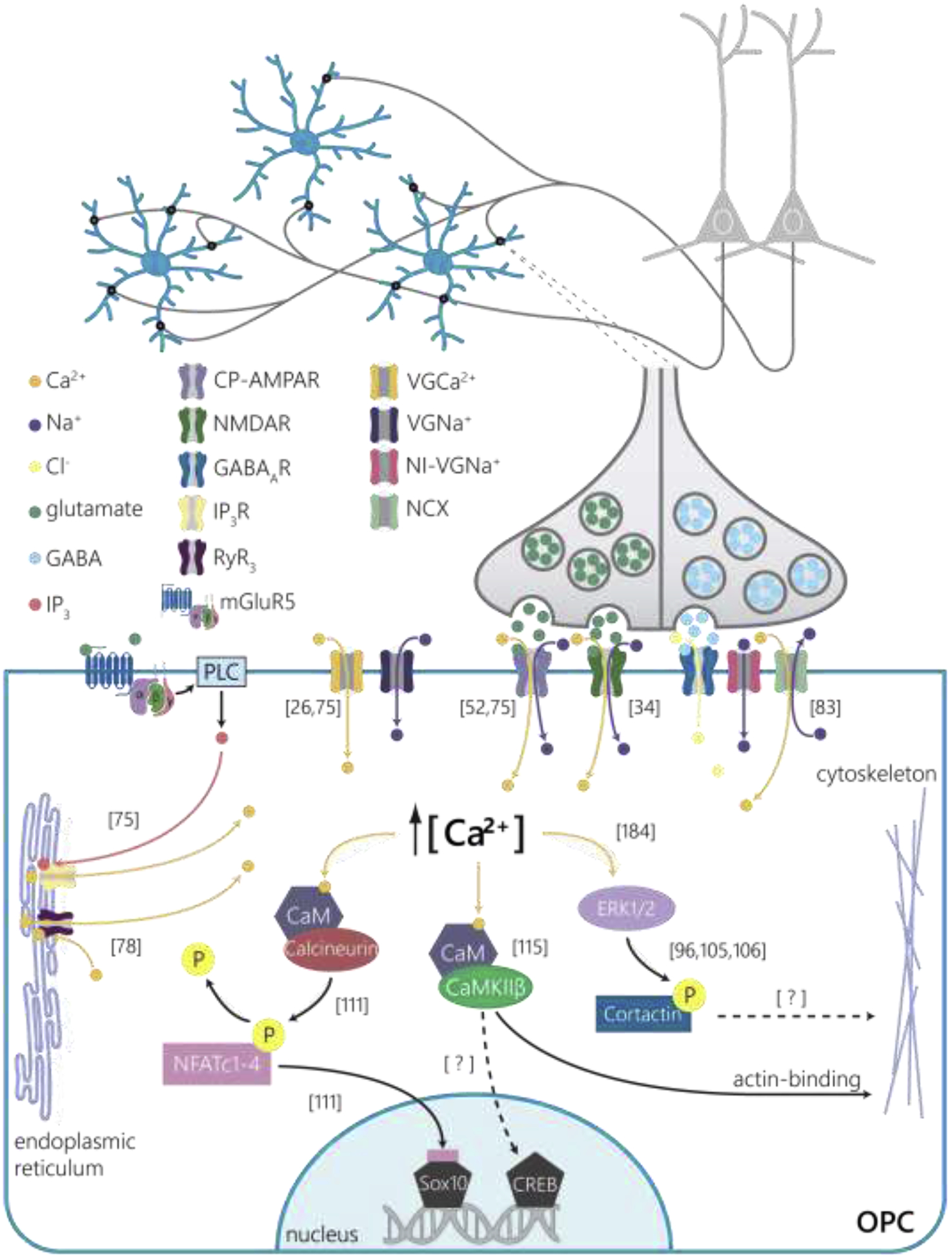
OPCs tile the CNS and engage in dynamic bidirectional signaling with neurons throughout life (top). Both synaptic (right) and extrasynaptic (left) signals regulate the early stages of oligodendroglial maturation. Intracellular signaling pathways mediating calcium-dependent events in differentiating OPCs, starting with receptors, from left to right: Glutamate binds to group I metabotropic glutamate receptors (blue) and induces Gq translocation and activation of phospholipase C (PLC), which releases IP3 (red) to the cytoplasm where it binds to IP3 receptors on the endoplasmic reticulum to release intracellular calcium stores (orange) [75]. OPCs express voltage-gated calcium (orange) and sodium (purple) channels, which increase intracellular calcium concentration and contribute to rapid depolarization in response to neuronal activity [26,75]. OPCs express calcium-permeable AMPA receptors (light purple) that contribute to synaptically-evoked calcium events and their membrane expression is modulated by mGluR5 activation [52,75]. NMDA receptors (dark green) also respond to glutamate release and may contribute to changes in expression of CP-AMPA receptors in differentiating OPCs [34]. Synaptic GABA release stimulates GABAA receptors (blue), which increases intracellular calcium concentration by increasing a persistent sodium current through non-inactivating voltage gated sodium channels (red) that facilitate reversal activity of sodium/calcium exchangers (NCX, light green, [83]). Calcium-induced calcium release from endoplasmic reticulum stores via ryanodine receptor 3 (RyR3) activation contributes to changes in intracellular calcium, and application of the antagonist ryanodine inhibits OPC differentiation [78]. Calcium-dependent intracellular signaling pathways that modulate gene expression and cytoskeletal dynamics, starting from left: Calcium binds to calmodulin (purple) and activates the phosphatase calcineurin (red) that dephosphorylates NFAT proteins (pink) to stimulate their translocation to the nucleus [111]. NFAT proteins associate with the transcription factor Sox10 to relieve reciprocal repression of Nkx2.2 and Olig2 (not pictured) to facilitate OPC differentiation [111]. Calcium activates Ca2+/calmodulin-dependent protein kinase II (CaMKIIβ, green), which stabilizes the actin cytoskeleton through non-kinase catalytic binding and may regulate differentiation [115]. Whether CaMKIIβ affects oligodendroglial behavior through the phosphorylation of CREB or other transcription factors remains to be tested. Calcium modulates the kinase activity of extracellular signal-related kinases (ERK1/2, [184]) which phosphorylates cortactin and may drive Arp2/3 complex-dependent actin polymerization to facilitate process extension and branching during OPC differentiation [96,105,106].
The expression of both calcium-permeable and impermeable AMPA receptors in OPCs [28] allows these cells to rapidly respond to synaptic input through membrane depolarization and local calcium influx. Recent studies manipulating AMPA receptor expression within the oligodendrocyte lineage defined roles for AMPA receptor activation and subunit composition in the balance of proliferation of OPCs and survival of mature oligodendrocytes. Modifying the amino acid composition of the GluA2 subunit to increase the calcium-permeability of AMPA receptors enhanced OPC proliferation and decreased the number of mature oligodendrocytes [29]. Loss of AMPA receptor signaling via germ-line double and triple knockouts of the GluA2–4 subunits did not alter OPC proliferation, but also decreased the survival of mature oligodendrocytes [30]. OPCs also express NMDA receptors and their activation is thought to play distinct roles in the oligodendrocyte lineage following CNS injury [31–33], however, the necessity for NMDA-mediated currents in normal OPC development and maturation remains unclear due to compensation by calcium-permeable AMPA receptors, whose expression is enhanced following NMDA receptor ablation (Fig. 2, [34,35]). These studies highlight an important role for glutamate signaling via AMPA and NMDA receptor signaling in the proliferation of OPCs and generation of new oligodendrocytes and myelin in the adult brain.
At interneuron-OPC synapses, gamma-aminobutyric acid (GABA) release induces brief GABAA receptor-mediated membrane postsynaptic events [36,37]. These miniature postsynaptic events elicit depolarizing changes in membrane potential due to an increased reversal potential for chloride in OPCs (~−43mV). In the adult hippocampus, GABAergic synaptic transmission promotes the differentiation of neuronal progenitors by increasing intracellular calcium and expression of NeuroD [38]. Similarly, GABAergic signaling onto OPCs has physiological consequences, increasing OPC proliferation and suppressing oligodendrogenesis [39]. Whether synaptic or extrasynaptic GABA receptors drive these changes in OPC proliferation and differentiation may depend on brain region and developmental age [40,41].
The close association in the distribution of GABAergic neurons and OPCs in the cortex [42], suggests a unique connection between interneurons and OPCs. During development, the secretion of the cytokine fractalkine from newborn interneurons, enhances the generation of oligodendrocytes [43]. This intimate interaction between interneurons and oligodendroglia continues in the adult CNS as a large fraction of myelinated fibers in the cortex are axons of fast-spiking PV neurons in mice and humans [44–46]. Furthermore, enhancing the activity of PV neurons increases their axonal arborization as well as their myelination [47] suggesting a role of interneuron-oligodendroglia interactions in plasticity in the adult brain. These findings and others have led to a recent hypothesis that the myelination of fast-spiking parvalbumin interneurons could play an important role in the pathophysiology of schizophrenia [46]. Future work examining how GABAergic signaling acts in concert with glutamatergic signaling to balance levels of oligodendrocyte proliferation, differentiation, and myelination in the normal and diseased CNS will provide additional insights into the functions of interneuron-oligodendroglia interactions.
Neurotransmitters and other signaling molecules
Do oligodendrocyte lineage cells sense and respond to other signaling molecules in addition to those at synapses? Oligodendrocyte precursors express a wide array of neurotransmitter and neuroactive ligand receptors. In addition to neurotransmitter receptors to amino acids (glutamate and GABA), OPCs express receptors for other neuromodulators (ATP, acetylcholine, histamine, norepinephrine, serotonin, dopamine) and neuropeptides (substance P, angiotensin II, bradykinin). Activation of these receptors in OPCs elicits intracellular calcium signaling, suggesting that oligodendroglia can sense and respond to numerous neuronal activity-dependent signals [48]. Since this topic has been extensively reviewed previously [21], we will only briefly describe how these signaling molecules affect oligodendroglia.
Metabotropic glutamate receptors (mGluRs) represent an extrasynaptic mechanism to detect axonal glutamate release and modulate intracellular signaling [49,50]. They are expressed in the oligodendrocyte lineage and their receptor subtype expression varies with cell cycle stage [51]. Group I mGluR activation increased the expression of calcium-permeable AMPA receptors in OPCs while purinergic stimulation decreased their expression, implying that mGluR activation can modulate the sensitivity of OPCs to synaptic activation (Fig. 2, [52]). However, additional experiments are needed to further define the role of mGluRs in shaping oligodendrocyte precursor behavior.
Adenosine triphosphate (ATP) and its derivatives are released from active axons and bind to purinergic receptors on OPCs. In vitro studies showed that adenosine application to in vitro cultures increased OPC differentiation [53], and stimulated calcium transients and myelin protein translation in oligodendrocyte processes [54]. While the role of ATP and purinergic receptors in oligodendroglia is less understood in the intact nervous system, signaling via another nucleotide receptor, G protein-coupled receptor 17 (GPR17, [55]), has been shown to regulate oligodendroglia in the intact CNS. GPR17 is predominantly expressed within the oligodendrocyte lineage in the nervous system, specifically within premyelinating oligodendrocytes [56,57]. Data from overexpression and knockout studies in mice indicate that GPR17 signaling regulates the transition between premyelinating and mature oligodendrocytes by modulating the nuclear translocation of helix-loop-helix proteins ID2/4 [56], which have been previously shown to be potent inhibitors of oligodendrocyte differentiation [58,59].
Oligodendrocyte lineage cells also express both nicotinic and muscarinic acetylcholine receptors, which allow them to sense and respond to cholinergic neuromodulation [60]. Cell-specific ablation of the M1 muscarinic receptor within the oligodendrocyte lineage lead to accelerated remyelination in experimental models of multiple sclerosis (MS, [61]). This finding has additional clinical relevance as veterans affected by Gulf War Illness show changes in white matter volume due to exposure to the acetylcholinesterase inhibitor sarin gas [60,62]. Recent clinical trials showed efficacy of the remyelinating drug candidate clemastine fumarate, an antagonist of muscarinic acetylcholine receptors, in patients with multiple sclerosis [63–65].
Cultured OPCs respond to other neuromodulators, such as serotonin, with increases in intracellular calcium and changes in differentiation and myelination [48,66]. While gene expression databases indicate that the only serotonin receptor subunit with significant expression in oligodendroglia is the 5HT5a receptor [20,67], mice deficient in tryptophan hydroxylase 2, the rate-limiting enzyme in the synthesis of serotonin, have increases in OPC proliferation in the hypothalamus [68]. However, the effects of serotonergic neuromodulation on the oligodendrocyte lineage remains incompletely understood.
Less is known about the roles of other neuromodulator receptors expressed on OPCs. Fluorescent reporter lines indicate that adrenergic receptors localize to OPCs [69] and application of norepinephrine in vitro leads to increases in IP3 production and calcium concentration [70,71]. Additional work indicates that adrenergic receptor activation inhibits OPC proliferation, while increasing differentiation in vitro [72]. Although the data on dopaminergic modulation of oligodendroglia is limited, one study found that the D3 receptor is expressed in OPCs during peak myelination and application of the dopamine receptor agonist quinpirole decreased differentiation in vitro [73]. Additionally, dopamine D2 and D3 receptor agonists protected oligodendrocytes from oxidative stress following glutamate excitotoxicity and oxygen/glucose injury, suggesting a potential role for these receptors in regulating oligodendrocyte metabolic pathways [74].
The diverse array of signaling molecule receptors expressed on oligodendrocyte lineage cells clearly allows for highly sensitive detection of extrasynaptic signals released during neuronal firing. Future experiments examining the selectivity and integration of these varied signals and how they translate into changes in cellular behavior will provide clearer insights on how oligodendroglia respond to and interact with the complexity of neuronal networks in the intact CNS.
2. Effects of activity-dependent calcium signaling in oligodendroglia
Multiple sources of calcium
Calcium signaling is a dynamic second messenger system that may link extrinsic signals to complex intracellular changes within the oligodendrocyte lineage. Calcium events in oligodendrocyte lineage cells are varied in their spatiotemporal dynamics and can be derived from both intra- and extracellular sources. Haberlandt et al. [75] showed that calcium-permeable AMPA receptors, voltage-gated calcium channels (VGCCs), group I metabotropic glutamate-receptors, and calcium-induced calcium release from the endoplasmic reticulum (ER) contribute to stimulation-induced calcium transients in OPC processes in acute hippocampal slices (Fig. 2). In addition to group I mGluRs, activation of other Gq-coupled protein receptors, such as muscarinic acetylcholine receptors [76] and P2Y purinergic receptors [77], leads to increases in intracellular calcium via phospholipase C-mediated release of inositol 1,4,5-triphosphate (IP3) and binding to ER IP3 receptors (Fig. 2). A recent study also demonstrated that calcium release from intracellular ER stores via RyR3 receptors is important for OPC differentiation in vitro [78]. The role of intracellular calcium release in regulating OPC behavior in vivo has yet to be explored.
Extracellular and synaptic signals induce extracellular calcium entry into OPCs via VGCCs, ligand-gated neurotransmitter receptors, and other transmembrane proteins. VGCCs have been studied in the oligodendrocyte lineage as possible mediators of activity-dependent changes in cellular behavior. For example, conditional deletion of the L-type calcium channel Cav1.2 in OPCs reduced oligodendrocyte maturation and myelination in the postnatal brain, and, following cuprizone demyelination, impaired remyelination of the corpus callosum and cortex [79,80]. Calcium-permeable AMPA receptors are a major source of calcium entry in OPCs [81], and they express high levels of mRNA transcript for Gria2 [20,67,82], which encodes the calcium permeability-determining AMPA receptor subunit. OPCs also express NMDA receptors [20,67,82], and although it has been suggested that their expression is not required for normal OPC development [34] they may still contribute to activity-dependent calcium flux in these precursors, especially in the context of injury [31]. GABAA receptor activity on OPCs increases intracellular calcium independent of VGCCs through the generation of a persistent non-inactivating sodium current (NI-VGNa+) that leads to reversal activity of sodium/calcium exchangers (NCX, Fig. 2, [83]). Changes in OPC intracellular calcium concentration arise from a plethora of sources and can be driven by a variety of signaling molecules. The pathway-specific effects of these spatiotemporally diverse calcium signals represent an intriguing area for future research.
The cellular sources and signaling consequences for calcium are less understood in the mature oligodendrocyte. In myelinating oligodendrocytes, NMDA receptors are thought to mediate calcium accumulation in response to ischemia [31–33] and a recent study showed that electrical stimulation in ex vivo optic nerve preparations induced NMDA-mediated calcium increases in myelin sheaths ([84]). However, Hamilton et al. [85] challenged the notion that NMDA receptors mediate calcium currents in mature oligodendrocytes, and instead proposed a mechanism dependent on proton-gated, calcium permeable TRPA1 channels. Recently, Battefeld et al. [86] combined visually-guided patch clamp electrophysiology with high-speed calcium imaging in somatosensory cortical slices to show that calcium changes in mature myelin sheaths of cortical oligodendrocytes were due to mitochondrial release in response to increased metabolic demand. Determining the sources and mechanisms driving calcium events in mature oligodendrocytes has proven to be difficult, however recent work has shed light on the specific roles of calcium in the formation and maintenance of myelin sheaths in response to changes in neuronal activity.
Modulation of intracellular calcium by neuronal activity
Does the manipulation of neuronal activity directly modulate oligodendroglial calcium events? Sun et al. [87] used hippocampal slices to show that electrical stimulation of OPCs induces calcium elevations in processes that are dependent on dendritic VGCCs. Physiologically relevant stimulation levels rarely elicited major calcium events in these ex vivo experiments, while the application of 4-AP increased the OPC responses, implying a role for A-type potassium channels in gating the calcium response to glutamatergic signaling. In contrast, in vivo two-photon imaging in mice expressing GCaM6f in OPCs revealed robust calcium responses to odor stimulation in olfactory bulb glomeruli OPCs [88]. Glomerular OPC activation was odor-specific and the onset of calcium responses in OPC processes was temporally indistinguishable from the activation of the olfactory receptor neuron axon terminals. Future studies using longitudinal in vivo multi-color calcium imaging techniques to visualize neuronal and OPC calcium signaling simultaneously will help to elucidate the mechanistic links between neuronal activity, OPC calcium influx, and oligodendrocyte lineage cell behavior.
Recent studies have also applied in vivo calcium imaging in zebrafish to assess the role of calcium transients during oligodendrocyte maturation and myelin wrapping [89,90]. Krasnow and colleagues found calcium transients in the processes and the cell soma of premyelinating oligodendrocytes decreased during maturation into myelinating oligodendrocytes. Additional experiments used tetrodotoxin (TTX) to block action potential generation and showed that a significant proportion of oligodendrocyte calcium events are dependent on neuronal activity. However, it is important to note that since oligodendroglia express tetrodotoxin-sensitive voltage-gated sodium channels [20,67,82], utilization of pharmacological methods to block neuronal activity (i.e. TTX) may also have effects on oligodendroglial ion balance and cell maturation. An emerging hypothesis is that high-frequency, short duration microdomain calcium events facilitate internode growth, while long-lasting calcium bursts induce sheath retraction during myelin development. To explore possible mechanisms of calcium-dependent sheath dynamics, Baraban et al. [89] showed that inhibiting calpain proteases increased the number of internodes produced by each oligodendrocyte, implicating calcium-induced proteolysis in the retraction of developing sheaths. Whether this calcium-dependent sheath remodeling is due to changes in neuronal activity or other metabolic processes [86] remains unknown. Future studies using fluorescent indicators for metabolic molecules may explore whether calcium signaling in myelin sheaths increases in response to metabolic demand.
Active regulation of cytoskeletal dynamics in oligodendrocyte lineage cells
What are the downstream intracellular targets of activity-dependent changes in calcium concentration? Calcium modulates multiple pathways including those that regulate cytoskeletal dynamics providing a potential link between extrinsic signals and different stages of cellular remodeling. Live imaging studies showed that OPCs in zebrafish spinal cord and adult mouse cortex extend ramified processes with dynamic filopodia-like structures that undergo extensive process reorganization [91,92]. Pharmacological disruption of microfilaments and microtubules in oligodendrocytes in vitro decreased process outgrowth and branching [93]. Pre-oligodendrocyte growth cones were shown to sense and retract from non-permissive substrates in vitro [94]. Determining the mechanisms governing activity- and calcium-dependent regulation of the reorganization of the cytoskeleton will greatly improve our understanding of the effects of neuron-oligodendroglia interactions on cellular dynamics.
OPC process extension and initial ensheathment is driven by F-actin polymerization, while later stages of myelin wrapping are dependent on actin depolymerization and retraction [95,96]. Oligodendrocyte lineage cells express the major proteins involved in F-actin-polymerization-driven protrusion (Arp2/3, N-WASP, WAVE, rhoGTPases) and they localize to the leading edges of extending OPC and oligodendrocyte processes [97]. Disruption of actin polymerization within oligodendroglia has provided important insights into OPC behavior and myelination. Pharmacological inhibition of N-WASP with wiskostatin induced filopodia and process retraction in OPCs and lead to a decreased number of axons selected for nascent ensheathment in the intact optic nerve [97]. Expression of a dominant negative form of CNS WAVE1 impaired process and lamellipodia outgrowth in vitro, and WAVE1 mutant mice have a hypomyelination phenotype in the corpus callosum and optic nerve [98]. In order to explore cytoskeletal dynamics during OPC differentiation in an unbiased manner, a recent study by Azevedo et al. [99] used whole-transcriptome analysis of soma-detached OPC membrane protrusions and live F-actin imaging across different stages of oligodendrocyte differentiation in vitro. Polymerization and crosslinking/anchoring proteins were highly expressed during early stages of oligodendrocyte maturation, while depolymerization and capping proteins were upregulated at later stages of OPC differentiation. In addition, they found RNAi-mediated knockdown of the actin-regulating protein Jmy disrupted the transition from protrusion remodeling to formation of the myelin membrane. Overall, these findings provided extensive insights into the roles of cytoskeletal remodeling in the oligodendrocyte lineage, yet it remains unclear whether extrinsic neuronal signals drive these intracellular processes.
Neuronal activity-dependent changes in intracellular calcium may modulate cytoskeletal dynamics in oligodendroglia in a similar manner to neuronal dendritic spines. Calcium influx through calcium-permeable AMPA receptors, which are expressed on neuronal dendrites and at neuron-OPC synapses [19,34,52,100], mediates the enlargement of dendritic spines during long-term potentiation (LTP) through rapid reorganization/stabilization of actin via cofilin and cortactin [101,102]. Furthermore, NMDA receptor-mediated calcium transients consolidate dendritic spine structure during LTP by driving the rapid association of the calcium-binding protein caldendrin with cortactin, protecting F-actin from cofilin-induced severing [103]. Modulating calcium influx in OPCs affects proliferation and differentiation (see above, [79,80]), however, whether these phenotypes are due to downstream effects of calcium-binding proteins on the cytoskeleton remains unknown. Calcium also mediates the phosphorylation of substrates by extracellular related kinases 1/2 (ERK1/2) that are recruited to the actin cytoskeleton and increase OPC filopodia growth during migration [104]. Since cortactin is a phosphorylation target of ERK1/2 [105] that activates Arp2/3 [106], and Zuchero et al. [96] showed the necessity for Arp2/3 complex-dependent actin assembly during process extension and axon ensheathment, future studies might address the role of activity-dependent, cortactin-mediated actin polymerization during OPC differentiation and process extension (Fig. 2).
Calcium-dependent regulation of gene expression in oligodendrocyte lineage cells
Calcium signaling mediates changes in gene expression in multiple brain cell types. In neurons, activity-dependent regulation of gene expression mediates functional changes underlying synaptic plasticity and other dynamic processes [107]. For example, glutamatergic transmission onto dendritic spines induces changes in intracellular calcium that are transmitted to the nucleus to regulate dendritic growth and complexity [108]. Might neuronal activity-dependent calcium events in oligodendrocyte lineage cells also signal to the nucleus to regulate gene expression? Calcium binding protein gene expression levels are enriched in OPCs and premyelinating oligodendrocytes [20,67,82] and glutamatergic synapses on OPCs are a prime structure through which neuronal activity might be coupled with transcriptional changes. In line with this hypothesis, glutamate stimulation of AMPA receptors on OPCs in vitro regulates immediate early gene expression by increasing intracellular calcium [109]. Single-cell analysis of transcriptional states in the visual cortex indicated that oligodendrocyte lineage cells have transcriptional responses to light exposure, with 33 genes differentially expressed in response to visual activity [67]. Whether these gene expression changes regulate the increased OPC differentiation and maturation following sensory enrichment [9] remains to be explored.
One pathway that may couple neuronal activity-induced calcium changes with oligodendrocyte gene expression is the nuclear factor of activated T-cells (NFAT)/calcineurin nuclear signaling pathway. Mice lacking calcineurin in neural crest cells have deficits in Schwann cell differentiation and peripheral myelination that are mediated by a disruption of calcium-induced activation of NFATc3/4 [110]. A recent study by Weider et al. (Fig. 2, [111]) presented NFAT/calcineurin signaling as a novel pathway through which changes in intracellular calcium might modulate the expression of genes related to oligodendrocyte differentiation and myelination. The authors used in vitro and in vivo methods to demonstrate that NFAT proteins are targeted by Sox10 to regulate cross-repression of Olig2 and Nkx2.2 and facilitate oligodendrocyte differentiation. Conditional deletion of calcineurin using either Sox10-Cre or CNP1-Cre mice had similar effects on oligodendrocyte number, which implies that calcineurin expression is not required for the initial stages of OPC proliferation, development, or cell death.
NFATs play important roles in the activation of T-lymphocytes [112], and may be potential drug targets for the treatment of MS. Dietz et al. [113] explored the role of NFATc1 and 2 in the experimental autoimmune encephalomyelitis mouse model of MS where demyelination is initiated by T cell infiltration of the CNS. Genetic ablation of NFATc1 and 2 blocked immune cell infiltration into the CNS and decreased EAE clinical scores, suggesting that NFAT antagonism may be an immunosuppressive option to prevent inflammatory attacks in MS. Together, these studies indicate that NFAT activation and/or overexpression may actually increase remyelination via transcriptional regulation of OPC differentiation, and therefore a combinatorial, cell- and isoform-specific approach to NFAT modulation may 1) decrease T cell activation and infiltration, and 2) increase remyelination through OPC differentiation.
Another potential pathway that could link neuronal activity-induced calcium changes with oligodendrocyte gene expression is the calcium/calmodulin-dependent protein kinase pathway. Oligodendroglia express calcium/calmodulin-dependent protein kinase type II subunit β (CAMKIIβ), an important molecular mediator of long-term potentiation and neuronal plasticity [114]. A recent study used in vitro methods combined with genetic knockout strategies to demonstrate that CAMKIIβ is important for cytoskeletal remodeling during differentiation and myelin thickness in the intact CNS (Fig. 2, [115]). Further research into the roles of calcium binding proteins in the oligodendrocyte lineage may provide a link between activity-dependent signaling and nuclear gene expression regulating oligodendrocyte maturation.
Activity-dependent growth factor signaling pathways that modulate oligodendrocyte maturation
Multiple growth factors regulate essential cellular processes that underlie proliferation, differentiation, and myelination in the oligodendrocyte lineage. Overexpression of insulin-like growth factor-1 (IGF-1) induces a hypermyelination phenotype [116] through the activation and multiplexed signaling of the PI3K/AKT/mTOR pathway. This atypical serine/threonine kinase, mechanistic target of rapamycin (mTOR), acts through binding two downstream proteins (Raptor and Rictor) to modulate differentiation and myelination [117–120]. BDNF-TrkB signaling in oligodendrocyte precursors activates the ERK/MAPK signaling cascade and increases differentiation and myelination through the action of ERK1/2 [121]. Sustained phosphorylation of ERK1/2 within mature oligodendrocytes resulted in a global hypermyelination phenotype that showed faster conduction speeds in the auditory brainstem [122,123] implying that mature oligodendrocytes can modulate the extent of their myelination.
Certain types of growth factors are released from neurons in an activity-dependent manner, suggesting that their effects on oligodendroglia are additional mechanisms through which neuronal activity modulates oligodendroglial behavior (Fig. 3, [124,125]). In vitro data in cultured cortical neurons suggested that NMDA receptor-mediated calcium events increase phosphorylation of AKT, and slice physiology of ventral tegmental area neurons showed that mGluR1-dependent long-term depression was blocked by rapamycin application, implying a role for the mTOR pathway in activity-dependent receptor expression and plasticity [126,127]. However, the effects of activity-dependent calcium signaling on the PI3K/AKT/mTOR and other growth factor pathways in the oligodendrocyte lineage remain to be tested.
Figure 3 |. Axonal activity mediates mature oligodendrocyte integration.
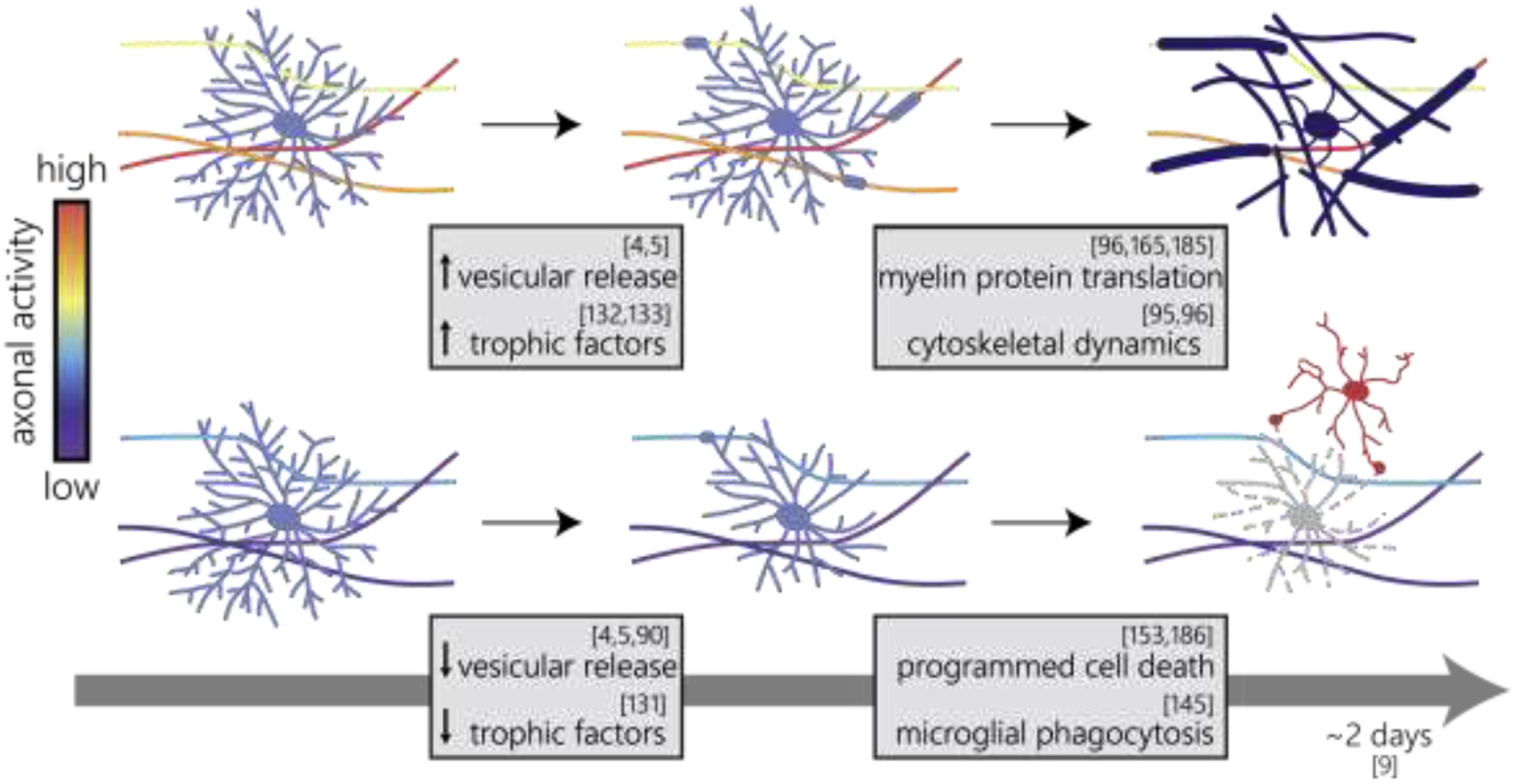
Adult differentiating oligodendrocytes remain in the premyelinating stage for ~2 days [9], during which they integrate as mature oligodendrocytes (top) or degenerate (bottom). High axonal activity (top) increases vesicular release [4,5], and the release of growth factors [132,133] to facilitate the initiation of myelination and integration into neuronal circuits. During the later stages of mature oligodendrocyte integration (top right), sustained axonal activity may upregulate local myelin protein translation [96,165,185] and drive actin depolymerization-driven myelin wrapping [95,96]. Decreasing axonal activity (bottom, left) reduces vesicular release [4,5,90] and the release of growth factors [131], which limits myelin initiation and integration. If appropriate myelination cues are not received during this short period (bottom, right), programmed cell death pathways are activated [153,186] and oligodendroglia degenerate and are phagocytocized by microglia (red, [145]).
Bidirectional interactions between growth factor and neurotransmitter signaling cascades in oligodendroglia may further regulate the detection of and response to neuronal activity. Lundgaard et al. [128] showed that the application of neuregulin or BDNF in vitro induces a switch in oligodendrocytes to an NMDA receptor-dependent myelination state. These data, combined with the finding that social isolation leads to decreased oligodendrocyte neuregulin-1/ErbB3 signaling and hypomyelination of the prefrontal cortex [129], point to a mechanism in which neuronal activity-dependent release of growth factors gates the responses of oligodendroglia to neurotransmitter release. A related line of research has emerged on the effects of methotrexate chemotherapy on glial function, activity-dependent myelination, and cognition [130]. In this model of chemotherapy-related cognitive impairment, activated microglia reduce BDNF expression, which leads to impairments in activity-dependent oligodendrocyte lineage cell proliferation, differentiation, and myelination [131]. Mice that conditionally lack expression of the TrkB receptor in OPCs (Pdgfrα-creERT2; TrkBfl/fl) or activity-dependent transcription of BDNF (BDNFTMKI) had impaired activity-dependent myelination in the premotor circuit and behavioral deficits in a modified novel object recognition test [131]. These studies, combined with previous work that linked BDNF signaling through TrkB receptors to later stages of myelin wrapping (Fig. 3, [132,133]), suggest that neuron-oligodendroglia BDNF signaling regulates multiple stages of oligodendrogenesis and myelination. Identification of the neuron-derived signals and subsequent activation of intracellular signaling cascades in oligodendroglia is an exciting focus of ongoing research in the field and will likely yield key insights into the molecular mechanisms underlying activity-dependent myelination.
3. Effects of neuronal activity on oligodendrocyte lineage cellular behavior
OPC motility and proliferation
The migration and cell division of OPCs can be modulated by extrinsic factors throughout life. During development, multiple waves of OPCs migrate from the ventricular zones to populate the telencephalon [134]. Wnt pathway activation enables this migration by driving physical interactions between OPCs and the endothelial cells lining the blood vessels [135]. Migrating OPCs extend growth-cone like structures to sense and respond to a multitude of chemotactic signals including growth factors, cell adhesion molecules, and extracellular matrix proteins [136,137]. Adult OPCs continue to migrate throughout life in response to local cues that include homotypic interactions, CNS injury, and neuronal activity [91,92,138]. Longitudinal in vivo two-photon imaging revealed that adult OPCs primarily undergo direct differentiation followed by proliferation of neighboring OPCs, indicating that population density is homeostatically maintained via balanced cell loss and renewal [92]. Although early studies showed a direct relationship between neuronal activity and OPC proliferation [2], the mechanisms underlying OPC migration have remained elusive. In vitro studies demonstrated that OPC migration speed and calcium transient frequency is modulated by the formation of an αv integrin/PLP protein complex elicited when glutamate binds to AMPA receptors on OPCs [139]. Additional studies using cell culture and slice preparation techniques have linked increased calcium signaling mediated by L- and T-type voltage gated calcium channels on OPCs with increased rates of migration [79,140].
OPCs express functionally active GABAB receptors that inhibit the production of cyclic adenosine monophosphate (cAMP) and in vitro experiments using a transwell microchamber assay showed that application of the specific agonist baclofen increased migration and proliferation [141]. OPC migration was also enhanced by NMDA-receptor activation and interaction with the Rac1-GEF and ERK signaling pathway induced cytoskeletal remodeling and actin dynamics [142]. Sensory deprivation of whisker input reduced thalamocortical inputs to OPCs, increased proliferation, and altered cell distribution during development of the barrel cortex, arguing for an inhibitory effect of excitatory input to OPCs on proliferation and migration in this brain region [143]. However, optogenetic stimulation of cortical layer V projection neurons in the premotor cortex induced a large OPC proliferation response within three hours of stimulation that was followed by an increased number of CC1/Edu-positive mature oligodendrocytes four weeks following stimulation [7]. These contrasting results highlight the significant region-, age- and cell developmental heterogeneity in receptor and ion channel expression on OPCs [26] which may underlie these different findings. In addition, the effects of neuronal stimulation on proliferation are dependent on the frequency and duration of firing indicating that neurons may tune myelination with different patterns of activity [6]. Thus, the effects of neuronal activity on OPC migration and proliferation likely depend on the cellular state, receptor and channel expression, and pattern/frequency of neuronal firing.
Integration of differentiating OPCs as mature oligodendrocytes
During development, the generation of new myelinating oligodendrocytes is an inefficient process. A large portion of OPCs that undergo differentiation fail to myelinate axons and subsequently degenerate (Fig. 3, [144,145]). The survival and integration of these differentiating OPCs is thought to be regulated by the availability of appropriate axons, competition for trophic factors, and neuronal activity (Fig. 3). Recent in vivo longitudinal two-photon imaging of the oligodendrocyte lineage in the somatosensory cortex of middle-aged mice showed that this phenomenon persists in the adult brain. Only 22% of OPCs that differentiated into premyelinating cells successfully integrated, and those that died persisted in the premyelinating state for ~2 days (Fig. 3, [9]). Cell death of newly differentiating oligodendrocytes is thought to be carried out by Bax/Bak-dependent apoptotic pathways (Fig. 3, [146]) and competition between promoting and inhibitory signals may establish a threshold for successful integration or degeneration. Thus, the short lifetime of premyelinating cells may be a sensitive period in which extrinsic cues, such as neuronal activity, are integrated to mediate the cellular transition to form a mature, myelinating oligodendrocyte. Indeed, whisker deprivation decreased the survival of recently divided OPCs in early postnatal pups [147] highlighting a role for neuronal activity in oligodendrocyte integration.
The ubiquitous distribution of OPCs throughout the entire CNS raises the question of whether regulation of OPC integration could shape the pattern of mature oligodendrocytes across the CNS. Studies using genetic strategies to increase axon size in the unmyelinated cerebellar molecular layer [148] or mislocalize myelinated axonal subtypes to unmyelinated superficial dorsal spinal cord [149] resulted in successful integration and ectopic myelination in these regions. Large-diameter axons can upregulate their expression of secreted signaling molecules like BDNF [148], suggesting that axon-specific cues could modulate the final steps of oligodendrocyte differentiation and integration to guide myelination (Fig. 3).
One potential negative regulator of late-stage differentiation and ensheathment is the G protein-coupled receptor 37 (GPR37), which is predominantly expressed in the nervous system, binds to the neuropeptides prosaptide and prosaposin, and is enriched in newly differentiated oligodendrocytes [82,150,151]. A recent report used genetic deletion to show that GPR37-signaling acts as a brake on integration that, when relieved, facilitates myelination through a cAMP-dependent mechanism that drives ERK1/2 translocation to the nucleus [151]. Interestingly, in striatal neurons, GPR37 negatively regulates the surface expression of the adenosine A2A receptor [152], suggesting that GPR37 regulation of adenosine receptor activity is an additional potential mechanism of neuronal activity-dependent regulation of oligodendrocyte integration.
Determining the intracellular pathways driving programmed cell death of premyelinating cells may reveal important insights into the extrinsic and intrinsic regulation of mature oligodendrocyte integration. Sun et al. [153] recently identified transcription factor EB (TFEB), a master regulator of lysosome biosynthesis, as an additional regulator of oligodendrocyte integration. Cell-specific deletion of TFEB increased the survival of premyelinating cells by preventing apoptosis and induced ectopic myelination in the cerebellar molecular layer, without affecting axon caliber (Fig. 3, [153]). Whole transcriptome analysis of premyelinating cells from TFEB conditional knockout mice showed that TFEB expression may also interact with the AKT/mTOR and Wnt pathways, both of which modulate mature oligodendrocyte differentiation and integration [120,153,154]. Additionally, lysosomal calcium signaling promotes the nuclear translocation of TFEB via calcineurin dephosphorylation to regulate autophagic cell death in vitro (Fig. 4, [155]). Thus, defining the interplay between activity-dependent intracellular calcium events and programmed cell death pathways may provide insights into the mechanisms driving integration of newly differentiated oligodendrocytes.
Effects of neuronal activity on myelin wrapping
Oligodendrocyte differentiation and myelination are incredible biological processes involving extensive cytoskeletal rearrangement and production of specialized membrane. As OPCs differentiate they transition from a state of actin polymerization-driven process protrusion to actin depolymerization/redistribution at the leading edge of the nascent myelin sheath [95,99]. In vitro live imaging studies showed that after axonal contact, the leading edge of the oligodendrocyte process wraps the axon and new layers extend laterally as they are added to the sheath [156,157]. In vivo live imaging studies in zebrafish have provided insights into how axons are selected for myelin ensheathment. Hines et al. [4] demonstrated that, while the targeting of axons for ensheathment is unchanged in the absence of vesicular release, silencing VAMP2-dependent exocytosis strongly decreased the growth and stabilization of initiated myelin sheaths (Fig. 3). A complementary study showed that blocking axonal vesicular release reduced the number of myelinated axons in the ventral spinal cord, while increasing neuronal activity with the GABAA-receptor antagonist PTZ increased the number of myelin sheaths created by individual oligodendrocytes (Fig. 3, [5]). Furthermore, the role of activity in regulating myelination is dependent on neuronal cell type. Kouldelka et al. [158] showed cell-specific blockade of vesicular release significantly decreases the myelination of reticulospinal axons, yet has little effect on myelination of commissural primary ascending axons in the developing zebrafish spinal cord. Elucidating the axon-specific cues that regulate selective myelination will be of great importance in understanding the regulation of myelination in neural circuits.
The identity of neuron-derived signals that regulate axon-specific myelination likely include both stimulating factors released from axons as well as inhibitory signals that reduce myelination. For example, next-generation sequencing of cultured spinal cord and dorsal root ganglion neurons resulted in the identification of neuronal junction adhesion molecule 2 (JAM2) as a somatodendritically-expressed inhibitory molecule that prevents myelination of non-axonal neuronal structures [159]. Might neuronal activity regulate the expression of axonally-expressed inhibitory molecules to facilitate axon-specific, temporally-controlled myelination? The proteolytic cleavage of synaptic cell adhesion molecules (CAMs) is regulated throughout life to modulate synapse formation and plasticity [160], while overexpression of a membrane-bound extracellular domain of cell adhesion molecule 4 (Cadm4) in oligodendrocytes increases the number of myelin initiation sites on axons [161]. A recent report showed that axons cluster synaptic release machinery at sites of myelination during zebrafish development, and the oligodendrocyte-specific expression of dominant negative synaptogenic adhesion proteins modulated sheath number and individual sheath length [162]. Future studies exploring the extent to which neuronal activity drives the local translation and modification of cell adhesion molecules in both axons and oligodendrocytes to facilitate oligodendrocyte process targeting and selective myelination will provide valuable insights.
Cytoplasmic channels transiently open during myelin sheath formation allow for membrane trafficking to the leading edge of the myelin sheath, which may be regulated by axonal signals that drive reorganization of the actin cytoskeleton [156]. Interestingly, the expression of myelin basic protein (MBP) facilitates axon wrapping as MBP binds to PI(4,5)P2 and induces the release of gelsolin and cofilin from the membrane (Fig. 4, [96]). Gelsolin and cofilin are calcium-dependent actin severing proteins that induce actin depolymerization [163,164] arguing that activity-dependent myelin wrapping may be driven by local calcium elevations in oligodendrocyte processes (Fig. 4). In cultured dorsal root ganglion neurons, extrasynaptic release of glutamate increases local synthesis of MBP through Fyn-kinase dependent signaling (Fig. 4, [54]) and recent data suggests that neuronal activity may drive localized mRNA trafficking and expression within nascent myelin sheaths [165]. Whether these mechanisms contribute to the maintenance or the modulation of myelin thickness [7,129,131,166] of mature myelin sheaths remains unclear.
Figure 4 |. Calcium signaling in mature oligodendrocytes.
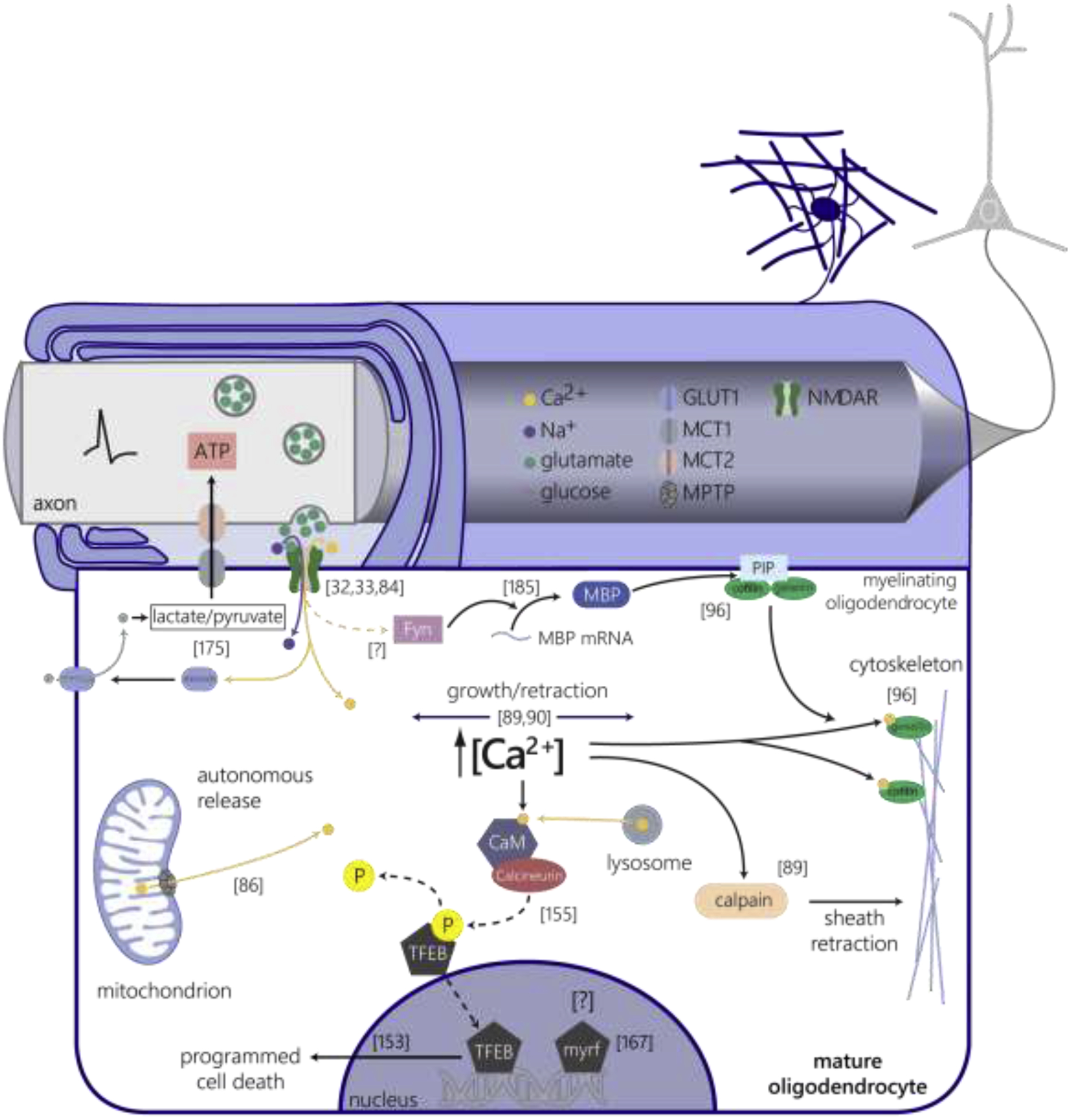
Dynamic bidirectional signaling between axons and mature oligodendrocytes regulates metabolic support, local protein translation, and gene expression. Mature oligodendrocytes sense and respond to neuronal glutamate release through NMDA receptors (green) expressed on the membrane in the periaxonal space [32,33,84]. A proportion of calcium elevations in mature myelin are derived from autonomous release through the mitochondrial permeability transition pore (MPTP, grey) in response to high mitochondrial respiration and production of ATP [86]. NMDA receptor-mediated calcium events increase the expression of the GLUT1 glucose transporter (light blue) on oligodendrocytes, which increases glucose (grey) entry and facilitates the delivery of lactate and pyruvate to active axons through monocarboxylate transporters 1 and 2 (grey, pink, [175]). Activity-dependent glutamatergic vesicle release increases the local translation of myelin basic protein (MBP) via a Fyn kinase-dependent mechanism [185], yet it is unknown whether changes in intracellular calcium modulate Fyn kinase activity to mediate this process. MBP binds to phosphatidylinositol 4,5-bisphosphate (PIP2) to release the calcium-dependent cytoskeletal reorganizing proteins gelsolin and cofilin from the membrane to induce actin depolymerization during myelin wrapping [96]. Neuronal activity-dependent elevations in intra-sheath calcium concentration regulate sheath growth and retraction in zebrafish development [89,90]. Oligodendrocyte-specific inhibition of calpain proteases increases the number of myelin sheaths produced, implicating calcium-induced proteolysis in sheath retraction [89]. Lysosomal calcium signaling activates calcineurin, which dephosphorylates the transcription factor EB (TFEB) and induces nuclear translocation to regulate autophagy [155]. TFEB regulates mature oligodendrocyte integration via activation of apoptotic pathways [153], yet it is unknown whether this process is regulated by intracellular calcium-calcineurin signaling. Mature oligodendrocyte-specific genetic deletion of the membrane-associated transcription factor myelin regulatory factor (myrf) results in oligodendrocyte degeneration [167], yet whether activity-dependent axonal signals regulate gene expression to support myelin maintenance or sheath remodeling is unknown.
Recent work has implicated myelin regulatory factor (Myrf) in the maintenance of mature myelin sheaths as conditional genetic ablation of this transcription factor results in the progressive degeneration of mature oligodendrocytes and axonal damage [167]. Whether axonal signals regulate the activity of Myrf to maintain myelin integrity and/or facilitate myelin remodeling over time remains to be tested (Fig. 4). Taken together with the recent calcium imaging data in developing myelin sheaths [89,90], these data suggest a model in which activity-dependent regulation of the expression of MBP and other myelin proteins, in concert with increases in intracellular calcium concentration, drives the initiation of actin depolymerization, wrapping, and maintenance of myelination of active axons (Fig. 4).
Oligodendrocyte-neuron signaling and metabolic support of active axons
Active axons require abundant metabolic energy to drive transporters and maintain ion concentration gradients essential for action potential firing [168]. Do oligodendrocytes support axonal metabolism in response to changes in neuronal activity? In myelinated axons, the area of axonal membrane through which energy metabolites and other molecules can be exchanged with the extracellular milieu is low, positioning oligodendrocytes as potential providers of axonal metabolic support through trans-myelin transport of energetic molecules [169]. Axons from the optic nerve use lactate as an energy source to sustain excitability when they are energy deprived [170], and in vivo voltage-sensitive dye imaging indicated that lactate was oxidized as an alternative to glucose during cortical hypoglycemia [171]. Although the role of oligodendrocytes in axonal metabolic support is well established, recent research has added considerably to our understanding of the molecular mechanisms involved in this process.
To determine whether oligodendrocytes play a role in metabolic support of axons, Fünfschilling and colleagues [172] generated conditional Cox10 knockout mice that have disrupted mitochondrial function in Schwann cells and oligodendrocytes. Increased rates of glycolysis in these mutant mice supplied metabolic products, such as lactate, to support energy-deprived axons indicating that axon-oligodendrocyte coupling has important physiological functions. Furthermore, oligodendrocyte-specific deletion of monocarboxylate transporter-1, the primary lactate transporter expressed in glia, resulted in pronounced axonal degeneration and neuronal death in the optic nerve and corpus callosum [173]. These studies suggest an integral role of glycolytic lactate in oligodendrocyte-neuron metabolic support. Recent experiments showed that bath perfusion of lactate was unable to rescue compound action potentials (CAPs) in corpus callosal slices during exogenous glucose deprivation, yet filling individual oligodendrocytes with glucose prevented CAP loss and was dependent on network spread through intact oligodendrocyte gap junctions [174]. These data suggest that the production and shuttling of both lactate and glucose by oligodendrocytes are essential to maintain axonal integrity and action potential firing, and metabolic processes used likely vary by brain region and developmental state [174].
Might mature oligodendrocytes detect and respond to glutamate release in order to deliver lactate and other energetic molecules to axons in need? A recent study by Saab et al. [175] showed that glutamatergic activation of NMDA receptors increases trafficking of the glucose transporter GLUT1 to the myelin membrane, which in turn enhances glucose transport into oligodendrocytes to facilitate the transport of lactate/pyruvate to the axon (Fig. 4). Since the activity of astrocytic GLUT1 is also strongly increased by glutamate release [176], and oligodendrocytes and astrocytes are coupled via gap junctions [177,178], these two cell types likely form a ‘panglial’ network that senses and responds to the energetic needs of active axons. Monitoring the transport of energetic molecules simultaneously in these two cell types in response to changes in neuronal activity may elucidate combinatorial cellular mechanisms of axonal support.
Roles of neuronal activity in the recovery from demyelinating disorders
Specific responses of oligodendrocyte lineage cells to changes in neuronal activity and neural plasticity following demyelinating injury or disease may be essential for remyelination and functional recovery. Following demyelination, transient synapses are formed on newly generated OPCs within the demyelinated lesion, and the proportion of OPCs innervated by glutamatergic axons is phasic during the remyelination process [179,180]. Gautier et al. [138] oligodendrocyte precursors to the site of injury and directed their differentiation into mature oligodendrocytes. In other injury found that glutamate released from demyelinated axons onto OPC AMPA receptors recruited models such as hypoxia-induced diffuse white matter injury, a loss of GABAA-mediated signaling onto OPCs resulted in increased proliferation, delayed maturation, and dysmyelination [39]. Blockade of GABA uptake or catabolism following hypoxia promoted the formation of myelinating oligodendrocytes suggesting that modulation of neuron-oligodendrocyte signaling may have therapeutic benefits [39]. In line with this hypothesis, blocking neuronal vesicular release, AMPA/KA, or NMDA receptors following lysolecithin-induced demyelination decreases remyelination of damaged white matter [128,138]. While it is clear that neuronal input onto oligodendroglia is essential for proper remyelination responses, future studies exploring the effects of region- and timing-dependent manipulations of neuronal activity on oligodendrocyte lineage cell behavior following injury will provide important insights into the ideal neural circuit targeting and stimulation parameters required for recovery from demyelinating injury.
Another route through which neuronal activity may control oligodendroglia injury responses is the activity-dependent upregulation of intracellular signaling molecules. For example, activity modulates the expression and release of Wnts in neurons [181] and may play a role in pathophysiological dysregulation of this neuron-oligodendroglia signaling in MS lesions. Elegant work showed that OPCs in demyelinated lesions specifically upregulate the Wnt pathway mediator transcription factor 4 (Tcf4) [154] and mice that have excessive Wnt activation due to conditional knockout of adenomatous polyposis coli (APC) show perivascular clustering of OPCs that disrupts the blood brain barrier. Thus, the complex interplay between neuronal activity, oligodendroglial intracellular signaling, and Wnt-mediated interactions with the vasculature may regulate the remyelination response and/or pathophysiology in demyelinating diseases.
Recently researchers have harnessed technological advances in specific neuronal stimulation techniques to manipulate neuronal activity during remyelination. Ortiz et al. [182] used repeated optogenetic stimulation of demyelinated corpus callosal fibers to facilitate OPC proliferation and increase remyelination following lysophosphatidylcholine-induced demyelinating injury. Li et al. [183] used epidural electrical stimulation of the primary motor cortex to increase remyelination and functional recovery after spinal cord injury (SCI). In the latter study, biotinylated dextran amine tracing of descending cortical inputs showed an increase in the number of axon-OPC synapses in the dorsal spinal cord. Taken together, these studies suggest an integral role for neuronal activity in the regulation of oligodendroglia during remyelination. Future research will determine whether behavioral interventions can harness the effects of neuronal activity and learning-induced activity-dependent myelination after injury [10,11] to promote functional recovery.
Conclusions and future directions
Communication between neurons and oligodendrocyte lineage cells is essential for proper brain-wiring and healthy neural function. Over the past few decades the mechanisms oligodendroglia use to sense and respond to neuronal activity have been characterized, and the links between neuronal activity and changes in oligodendroglia intracellular signaling, gene expression, and cellular behavior are beginning to emerge. Future studies manipulating specific intracellular targets to control the detection and response of oligodendrocyte lineage cells to activity will greatly facilitate our understanding of these complex interactions. Furthermore, modulation of neuron-oligodendrocyte signaling and in vivo monitoring of cellular behavior will provide invaluable insights for understanding learning-induced plasticity in the adult brain and the treatment of demyelinating injury and disease.
Acknowledgements
The authors would like to thank Clara Bacmeister and Helena Barr for critical evaluation and insightful comments on the manuscript. MAT is supported by the Neuroscience Graduate Training Grant (5T32NS099042-17). This work was supported by the Boettcher Foundation Webb-Waring Biomedical Research Award, the Whitehall Foundation, the Conrad N. Hilton Foundation (17324), and the National Multiple Sclerosis Society (RG-1701-26733) and NINDS (NS106432, NS115975) to EGH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hartline DK, Colman DR, Rapid Conduction and the Evolution of Giant Axons and Myelinated Fibers, Curr. Biol 17 (2007) R29–R35. 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- [2].Barres BA, Raff MC, Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons, Nature. 361 (1993) 258–260. 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- [3].Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C, Induction of myelination in the central nervous system by electrical activity., Proc. Natl. Acad. Sci. U. S. A 93 (1996) 9887–9892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B, Neuronal activity biases axon selection for myelination in vivo, Nat. Neurosci 18 (2015) 683–689. 10.1038/nn.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA, Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo, Nat. Neurosci 18 (2015) 628–630. 10.1038/nn.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nagy B, Hovhannisyan A, Barzan R, Chen T-J, Kukley M, Different patterns of neuronal activity trigger distinct responses of oligodendrocyte precursor cells in the corpus callosum, PLOS Biol. 15 (2017) e2001993 10.1371/journal.pbio.2001993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M, Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain, Science. 344 (2014) 1252304 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, Emery B, Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner, Nat. Commun 9 (2018) 306 10.1038/s41467-017-02719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE, Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex, Nat. Neurosci 21 (2018) 696–706. 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD, Motor skill learning requires active central myelination, Science. 346 (2014) 318–322. 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD, Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning, Nat. Neurosci 19 (2016) 1210–1217. 10.1038/nn.4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pan S, Mayoral SR, Choi HS, Chan JR, Kheirbek MA, Preservation of a remote fear memory requires new myelin formation, Nat. Neurosci (2020) 1–13. 10.1038/s41593-019-0582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Steadman PE, Xia F, Ahmed M, Mocle AJ, Penning ARA, Geraghty AC, Steenland HW, Monje M, Josselyn SA, Frankland PW, Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice, Neuron. 105 (2020) 150–164.e6. 10.1016/j.neuron.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yeung MSY, Zdunek S, Bergmann O, Bernard S, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Brundin L, Druid H, Frisén J, Dynamics of Oligodendrocyte Generation and Myelination in the Human Brain, Cell. 159 (2014) 766–774. 10.1016/j.cell.2014.10.011. [DOI] [PubMed] [Google Scholar]
- [15].Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F, Extensive piano practicing has regionally specific effects on white matter development, Nat. Neurosci 8 (2005) 1148–1150. 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- [16].Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H, Training induces changes in white matter architecture, Nat. Neurosci 12 (2009) 1370–1371. 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC, Local Brain Functional Activity Following Early Deprivation: A Study of Postinstitutionalized Romanian Orphans, NeuroImage. 14 (2001) 1290–1301. 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- [18].Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, Chugani DC, Makki M, Abnormal Brain Connectivity in Children After Early Severe Socioemotional Deprivation: A Diffusion Tensor Imaging Study, Pediatrics. 117 (2006) 2093–2100. 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- [19].Bergles DE, Roberts JD, Somogyi P, Jahr CE, Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus, Nature. 405 (2000) 187–191. 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- [20].Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, Wu JQ, An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex, J. Neurosci 34 (2014) 11929–11947. 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Larson VA, Zhang Y, Bergles DE, Electrophysiological properties of NG2+ cells: Matching physiological studies with gene expression profiles, Brain Res. 1638 (2016) 138–160. 10.1016/j.brainres.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chittajallu R, Aguirre A, Gallo V, NG2-positive cells in the mouse white and grey matter display distinct physiological properties, J. Physiol 561 (2004) 109–122. 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lin S-C, Huck JHJ, Roberts JDB, Macklin WB, Somogyi P, Bergles DE, Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum, Neuron. 46 (2005) 773–785. 10.1016/j.neuron.2005.04.025. [DOI] [PubMed] [Google Scholar]
- [24].Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE, Vesicular release of glutamate from unmyelinated axons in white matter, Nat. Neurosci 10 (2007) 321–330. 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vélez-Fort M, Maldonado PP, Butt AM, Audinat E, Angulo MC, Postnatal switch from synaptic to extrasynaptic transmission between interneurons and NG2 cells, J. Neurosci. Off. J. Soc. Neurosci 30 (2010) 6921–6929. 10.1523/JNEUROSCI.0238-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Spitzer SO, Sitnikov S, Kamen Y, Evans KA, Kronenberg-Versteeg D, Dietmann S, de Faria O, Agathou S, Káradóttir RT, Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age, Neuron. 101 (2019) 459–471.e5. 10.1016/j.neuron.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mount CW, Yalçın B, Cunliffe-Koehler K, Monje M, Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity, BioRxiv. (2019) 669572 10.1101/669572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Maldonado PP, Angulo MC, Multiple Modes of Communication between Neurons and Oligodendrocyte Precursor Cells, The Neuroscientist. 21 (2015) 266–276. 10.1177/1073858414530784. [DOI] [PubMed] [Google Scholar]
- [29].Chen T-J, Kula B, Nagy B, Barzan R, Gall A, Ehrlich I, Kukley M, In Vivo Regulation of Oligodendrocyte Precursor Cell Proliferation and Differentiation by the AMPA-Receptor Subunit GluA2, Cell Rep. 25 (2018) 852–861.e7. 10.1016/j.celrep.2018.09.066. [DOI] [PubMed] [Google Scholar]
- [30].Kougioumtzidou E, Shimizu T, Hamilton NB, Tohyama K, Sprengel R, Monyer H, Attwell D, Richardson WD, Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival, ELife. 6 (2017) e28080 10.7554/eLife.28080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Káradóttir R, Cavelier P, Bergersen LH, Attwell D, NMDA receptors are expressed in oligodendrocytes and activated in ischaemia, Nature. 438 (2005) 1162–1166. 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Salter MG, Fern R, NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury, Nature. 438 (2005) 1167–1171. 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- [33].Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK, NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia, Nature. 439 (2006) 988–992. 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- [34].De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE, NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination, J. Neurosci. Off. J. Soc. Neurosci 31 (2011) 12650–12662. 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guo F, Maeda Y, Ko EM, Delgado M, Horiuchi M, Soulika A, Miers L, Burns T, Itoh T, Shen H, Lee E, Sohn J, Pleasure D, Disruption of NMDA Receptors in Oligodendroglial Lineage Cells Does Not Alter Their Susceptibility to Experimental Autoimmune Encephalomyelitis or Their Normal Development, J. Neurosci 32 (2012) 639–645. 10.1523/JNEUROSCI.4073-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lin S, Bergles DE, Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus, Nat. Neurosci 7 (2004) 24–32. 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- [37].Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D, Glial cells are born with synapses, FASEB J. 22 (2008) 2957–2969. 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- [38].Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T, GABAergic Excitation Promotes Neuronal Differentiation in Adult Hippocampal Progenitor Cells, Neuron. 47 (2005) 803–815. 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- [39].Zonouzi M, Scafidi J, Li P, McEllin B, Edwards J, Dupree JL, Harvey L, Sun D, Hübner CA, Cull-Candy SG, Farrant M, Gallo V, GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury, Nat. Neurosci 18 (2015) 674–682. 10.1038/nn.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Orduz D, Maldonado PP, Balia M, Vélez-Fort M, de Sars V, Yanagawa Y, Emiliani V, Angulo MC, Interneurons and oligodendrocyte progenitors form a structured synaptic network in the developing neocortex, ELife. 4 (2015). 10.7554/eLife.06953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Balia M, Vélez-Fort M, Passlick S, Schäfer C, Audinat E, Steinhäuser C, Seifert G, Angulo MC, Postnatal Down-Regulation of the GABAA Receptor γ2 Subunit in Neocortical NG2 Cells Accompanies Synaptic-to-Extrasynaptic Switch in the GABAergic Transmission Mode, Cereb. Cortex 25 (2015) 1114–1123. 10.1093/cercor/bht309. [DOI] [PubMed] [Google Scholar]
- [42].Boulanger JJ, Messier C, Oligodendrocyte progenitor cells are paired with GABA neurons in the mouse dorsal cortex: Unbiased stereological analysis, Neuroscience. 362 (2017) 127–140. 10.1016/j.neuroscience.2017.08.018. [DOI] [PubMed] [Google Scholar]
- [43].Voronova A, Yuzwa SA, Wang BS, Zahr S, Syal C, Wang J, Kaplan DR, Miller FD, Migrating Interneurons Secrete Fractalkine to Promote Oligodendrocyte Formation in the Developing Mammalian Brain, Neuron. 94 (2017) 500–516.e9. 10.1016/j.neuron.2017.04.018. [DOI] [PubMed] [Google Scholar]
- [44].Micheva KD, Wolman D, Mensh BD, Pax E, Buchanan J, Smith SJ, Bock DD, A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons, ELife. 5 (2016) e15784 10.7554/eLife.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Micheva KD, Chang EF, Nana AL, Seeley WW, Ting JT, Cobbs C, Lein E, Smith SJ, Weinberg RJ, Madison DV, Distinctive Structural and Molecular Features of Myelinated Inhibitory Axons in Human Neocortex, ENeuro. 5 (2018) ENEURO.0297–18.2018. 10.1523/ENEURO.0297-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stedehouder J, Kushner SA, Myelination of parvalbumin interneurons: a parsimonious locus of pathophysiological convergence in schizophrenia, Mol. Psychiatry 22 (2017) 4–12. 10.1038/mp.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Stedehouder J, Brizee D, Shpak G, Kushner SA, Activity-Dependent Myelination of Parvalbumin Interneurons Mediated by Axonal Morphological Plasticity, J. Neurosci 38 (2018) 3631–3642. 10.1523/JNEUROSCI.0074-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bernstein M, Lyons SA, Möller T, Kettenmann H, Receptor-mediated calcium signalling in glial cells from mouse corpus callosum slices, J. Neurosci. Res 46 (1996) 152–163. . [DOI] [PubMed] [Google Scholar]
- [49].Ribeiro FM, Vieira LB, Pires RGW, Olmo RP, Ferguson SSG, Metabotropic glutamate receptors and neurodegenerative diseases, Pharmacol. Res 115 (2017) 179–191. 10.1016/j.phrs.2016.11.013. [DOI] [PubMed] [Google Scholar]
- [50].Spampinato SF, Copani A, Nicoletti F, Sortino MA, Caraci F, Metabotropic Glutamate Receptors in Glial Cells: A New Potential Target for Neuroprotection?, Front. Mol. Neurosci 11 (2018). 10.3389/fnmol.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Luyt K, Varadi A, Molnar E, Functional metabotropic glutamate receptors are expressed in oligodendrocyte progenitor cells, J. Neurochem 84 (2003) 1452–1464. [DOI] [PubMed] [Google Scholar]
- [52].Zonouzi M, Renzi M, Farrant M, Cull-Candy SG, Bidirectional plasticity of calcium-permeable AMPA receptors in oligodendrocyte lineage cells, Nat. Neurosci 14 (2011) 1430–1438. 10.1038/nn.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stevens B, Porta S, Haak LL, Gallo V, Fields RD, Adenosine: A Neuron-Glial Transmitter Promoting Myelination in the CNS in Response to Action Potentials, Neuron. 36 (2002) 855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD, Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons, Nat. Commun 6 (2015) 7844 10.1038/ncomms8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP, The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor, EMBO J. 25 (2006) 4615–4627. 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, Trapp BD, Karandikar NJ, Hsieh J, Lu QR, The oligodendrocyte-specific G protein–coupled receptor GPR17 is a cell-intrinsic timer of myelination, Nat. Neurosci 12 (2009) 1398–1406. 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Boda E, Viganò F, Rosa P, Fumagalli M, Labat-Gest V, Tempia F, Abbracchio MP, Dimou L, Buffo A, The GPR17 receptor in NG2 expressing cells: Focus on in vivocell maturation and participation in acute trauma and chronic damage, Glia. 59 (2011) 1958–1973. 10.1002/glia.21237. [DOI] [PubMed] [Google Scholar]
- [58].Kondo T, Raff M, The Id4 HLH protein and the timing of oligodendrocyte differentiation, EMBO J. 19 (2000) 1998–2007. 10.1093/emboj/19.9.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang S, Sdrulla A, Johnson JE, Yokota Y, Barres BA, A Role for the Helix-Loop-Helix Protein Id2 in the Control of Oligodendrocyte Development, Neuron. 29 (2001) 603–614. 10.1016/S0896-6273(01)00237-9. [DOI] [PubMed] [Google Scholar]
- [60].Fields RD, Dutta DJ, Belgrad J, Robnett M, Cholinergic signaling in myelination, Glia. 65 (2017) 687–698. 10.1002/glia.23101. [DOI] [PubMed] [Google Scholar]
- [61].Mei F, Lehmann-Horn K, Shen Y-AA, Rankin KA, Stebbins KJ, Lorrain DS, Pekarek K, A Sagan S, Xiao L, Teuscher C, von Büdingen H-C, Wess J, Lawrence JJ, Green AJ, Fancy SP, Zamvil SS, Chan JR, Accelerated remyelination during inflammatory demyelination prevents axonal loss and improves functional recovery, ELife. 5 (2016). 10.7554/eLife.18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Heaton KJ, Palumbo CL, Proctor SP, Killiany RJ, Yurgelun-Todd DA, White RF, Quantitative magnetic resonance brain imaging in US army veterans of the 1991 Gulf War potentially exposed to sarin and cyclosarin, NeuroToxicology. 28 (2007) 761–769. 10.1016/j.neuro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [63].Green AJ, Gelfand JM, Cree BA, Bevan C, Boscardin WJ, Mei F, Inman J, Arnow S, Devereux M, Abounasr A, Nobuta H, Zhu A, Friessen M, Gerona R, von Büdingen HC, Henry RG, Hauser SL, Chan JR, Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial, The Lancet 390 (2017) 2481–2489. 10.1016/S0140-6736(17)32346-2. [DOI] [PubMed] [Google Scholar]
- [64].Mei F, Fancy SPJ, Shen Y-AA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, Etxeberria A, Xiao L, Franklin RJM, Green A, Hauser SL, Chan JR, Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis, Nat. Med 20 (2014) 954–960. 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Deshmukh VA, Tardif V, Lyssiotis CA, Green CC, Kerman B, Kim HJ, Padmanabhan K, Swoboda JG, Ahmad I, Kondo T, Gage FH, Theofilopoulos AN, Lawson BR, Schultz PG, Lairson LL, A regenerative approach to the treatment of multiple sclerosis, Nature. 502 (2013) 327–332. 10.1038/nature12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fan L-W, Bhatt A, Tien L-T, Zheng B, Simpson KL, Lin RCS, Cai Z, Kumar P, Pang Y, Exposure to serotonin adversely affects oligodendrocyte development and myelination in vitro, J. Neurochem 133 (2015) 532–543. 10.1111/jnc.12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hrvatin S, Hochbaum DR, Nagy MA, Cicconet M, Robertson K, Cheadle L, Zilionis R, Ratner A, Borges-Monroy R, Klein AM, Sabatini BL, Greenberg ME, Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex, Nat. Neurosci 21 (2018) 120 10.1038/s41593-017-0029-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].van Lingen M, Sidorova M, Alenina N, Klempin F, Lack of Brain Serotonin Affects Feeding and Differentiation of Newborn Cells in the Adult Hypothalamus, Front. Cell Dev. Biol 7 (2019). 10.3389/fcell.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Papay R, Gaivin R, McCune DF, Rorabaugh BR, Macklin WB, McGrath JC, Perez DM, Mouse alpha1B-adrenergic receptor is expressed in neurons and NG2 oligodendrocytes, J. Comp. Neurol 478 (2004) 1–10. 10.1002/cne.20215. [DOI] [PubMed] [Google Scholar]
- [70].Cohen RI, Almazan G, Norepinephrine-stimulated PI hydrolysis in oligodendrocytes is mediated by alpha 1A-adrenoceptors, Neuroreport. 4 (1993) 1115–1118. [PubMed] [Google Scholar]
- [71].Kastritsis CH, McCarthy KD, Oligodendroglial lineage cells express neuroligand receptors, Glia. 8 (1993) 106–113. 10.1002/glia.440080206. [DOI] [PubMed] [Google Scholar]
- [72].Ghiani CA, Eisen AM, Yuan X, DePinho RA, McBain CJ, Gallo V, Neurotransmitter receptor activation triggers p27(Kip1)and p21(CIP1) accumulation and G1 cell cycle arrest in oligodendrocyte progenitors, Development. 126 (1999) 1077–1090. [DOI] [PubMed] [Google Scholar]
- [73].Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT, Identification of the Dopamine D3 Receptor in Oligodendrocyte Precursors: Potential Role in Regulating Differentiation and Myelin Formation, J. Neurosci 18 (1998) 5344–5353. 10.1523/JNEUROSCI.18-14-05344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rosin C, Colombo S, Calver AA, Bates TE, Skaper SD, Dopamine D2 and D3 receptor agonists limit oligodendrocyte injury caused by glutamate oxidative stress and oxygen/glucose deprivation, Glia. 52 (2005) 336–343. 10.1002/glia.20250. [DOI] [PubMed] [Google Scholar]
- [75].Haberlandt C, Derouiche A, Wyczynski A, Haseleu J, Pohle J, Karram K, Trotter J, Seifert G, Frotscher M, Steinhäuser C, Jabs R, Gray matter NG2 cells display multiple Ca2+-signaling pathways and highly motile processes, PloS One. 6 (2011) e17575 10.1371/journal.pone.0017575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Cohen RI, Almazan G, Rat oligodendrocytes express muscarinic receptors coupled to phosphoinositide hydrolysis and adenylyl cyclase, Eur. J. Neurosci 6 (1994) 1213–1224. 10.1111/j.1460-9568.1994.tb00620.x. [DOI] [PubMed] [Google Scholar]
- [77].Agresti C, Meomartini ME, Amadio S, Ambrosini E, Serafini B, Franchini L, Volonté C, Aloisi F, Visentin S, Metabotropic P2 receptor activation regulates oligodendrocyte progenitor migration and development, Glia. 50 (2005) 132–144. 10.1002/glia.20160. [DOI] [PubMed] [Google Scholar]
- [78].Li T, Wang L, Ma T, Wang S, Niu J, Li H, Xiao L, Dynamic Calcium Release From Endoplasmic Reticulum Mediated by Ryanodine Receptor 3 Is Crucial for Oligodendroglial Differentiation, Front. Mol. Neurosci 11 (2018). 10.3389/fnmol.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cheli VT, Santiago González DA, Namgyal Lama T, Spreuer V, Handley V, Murphy GG, Paez PM, Conditional Deletion of the L-Type Calcium Channel Cav1.2 in Oligodendrocyte Progenitor Cells Affects Postnatal Myelination in Mice, J. Neurosci 36 (2016) 10853–10869. 10.1523/JNEUROSCI.1770-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Santiago González DA, Cheli VT, Zamora NN, Lama TN, Spreuer V, Murphy GG, Paez PM, Conditional Deletion of the L-Type Calcium Channel Cav1.2 in NG2-Positive Cells Impairs Remyelination in Mice, J. Neurosci. Off. J. Soc. Neurosci 37 (2017) 10038–10051. 10.1523/JNEUROSCI.1787-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ge W-P, Yang X-J, Zhang Z, Wang H-K, Shen W, Deng Q-D, Duan S, Long-Term Potentiation of Neuron-Glia Synapses Mediated by Ca2+-Permeable AMPA Receptors, Science. 312 (2006) 1533–1537. 10.1126/science.1124669. [DOI] [PubMed] [Google Scholar]
- [82].Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcão A, Xiao L, Li H, Häring M, Hochgerner H, Romanov RA, Gyllborg D, Muñoz Manchado A, La Manno G, Lönnerberg P, Floriddia EM, Rezayee F, Ernfors P, Arenas E, Hjerling-Leffler J, Harkany T, Richardson WD, Linnarsson S, Castelo-Branco G, Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system, Science. 352 (2016) 1326–1329. 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tong X, Li X, Zhou B, Shen W, Zhang Z, Xu T, Duan S, Ca2+ signaling evoked by activation of Na+ channels and Na+/Ca2+ exchangers is required for GABA-induced NG2 cell migration, J. Cell Biol 186 (2009) 113–128. 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Micu I, Plemel JR, Lachance C, Proft J, Jansen AJ, Cummins K, Van JM, Stys PK, The molecular physiology of the axo-myelinic synapse., Exp. Neurol 276 (2016) 41–50. 10.1016/j.expneurol.2015.10.006. [DOI] [PubMed] [Google Scholar]
- [85].Hamilton NB, Kolodziejczyk K, Kougioumtzidou E, Attwell D, Proton-gated Ca2+-permeable TRP channels damage myelin in conditions mimicking ischaemia, Nature. 529 (2016) 523–527. 10.1038/nature16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Battefeld A, Popovic MA, de Vries SI, Kole MHP, High-Frequency Microdomain Ca2+ Transients and Waves during Early Myelin Internode Remodeling, Cell Rep. 26 (2019) 182–191.e5. 10.1016/j.celrep.2018.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sun W, Matthews EA, Nicolas V, Schoch S, Dietrich D, NG2 glial cells integrate synaptic input in global and dendritic calcium signals, ELife. 5 (2016) e16262 10.7554/eLife.16262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rungta RL, Chaigneau E, Osmanski B-F, Charpak S, Vascular Compartmentalization of Functional Hyperemia from the Synapse to the Pia, Neuron. 99 (2018) 362–375.e4. 10.1016/j.neuron.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Baraban M, Koudelka S, Lyons DA, Ca 2+ activity signatures of myelin sheath formation and growth in vivo, Nat. Neurosci 21 (2018) 19–23. 10.1038/s41593-017-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Krasnow AM, Ford MC, Valdivia LE, Wilson SW, Attwell D, Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo, Nat. Neurosci 21 (2018) 24–28. 10.1038/s41593-017-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kirby BB, Takada N, Latimer AJ, Shin J, Carney TJ, Kelsh RN, Appel B, In vivo time-lapse imaging shows dynamic oligodendrocyte progenitor behavior during zebrafish development, Nat. Neurosci 9 (2006) 1506–1511. 10.1038/nn1803. [DOI] [PubMed] [Google Scholar]
- [92].Hughes EG, Kang SH, Fukaya M, Bergles DE, Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain, Nat. Neurosci 16 (2013) 668–676. 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Song J, Goetz BD, Baas PW, Duncan ID, Cytoskeletal Reorganization during the Formation of Oligodendrocyte Processes and Branches, Mol. Cell. Neurosci 17 (2001) 624–636. 10.1006/mcne.2001.0974. [DOI] [PubMed] [Google Scholar]
- [94].Fox MA, Afshari FS, Alexander JK, Colello RJ, Fuss B, Growth conelike sensorimotor structures are characteristic features of postmigratory, premyelinating oligodendrocytes, Glia. 53 (2006) 563–566. 10.1002/glia.20293. [DOI] [PubMed] [Google Scholar]
- [95].Nawaz S, Sánchez P, Schmitt S, Snaidero N, Mitkovski M, Velte C, Brückner BR, Alexopoulos I, Czopka T, Jung SY, Rhee JS, Janshoff A, Witke W, Schaap IAT, Lyons DA, Simons M, Actin filament turnover drives leading edge growth during myelin sheath formation in the central nervous system, Dev. Cell 34 (2015) 139–151. 10.1016/j.devcel.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zuchero JB, Fu M-M, Sloan SA, Ibrahim A, Olson A, Zaremba A, Dugas JC, Wienbar S, Caprariello AV, Kantor C, Leonoudakis D, Leonoudakus D, Lariosa-Willingham K, Kronenberg G, Gertz K, Soderling SH, Miller RH, Barres BA, CNS myelin wrapping is driven by actin disassembly, Dev. Cell 34 (2015) 152–167. 10.1016/j.devcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Bacon C, Lakics V, Machesky L, Rumsby M, N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination, Glia. 55 (2007) 844–858. 10.1002/glia.20505. [DOI] [PubMed] [Google Scholar]
- [98].Kim H-J, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK, WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination, J. Neurosci. Off. J. Soc. Neurosci 26 (2006) 5849–5859. 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Azevedo MM, Domingues HS, Cordelières FP, Sampaio P, Seixas AI, Relvas JB, Jmy regulates oligodendrocyte differentiation via modulation of actin cytoskeleton dynamics, Glia. 66 (2018) 1826–1844. 10.1002/glia.23342. [DOI] [PubMed] [Google Scholar]
- [100].Cull-Candy S, Kelly L, Farrant M, Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond, Curr. Opin. Neurobiol 16 (2006) 288–297. 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- [101].Fortin DA, Davare MA, Srivastava T, Brady JD, Nygaard S, Derkach VA, Soderling TR, LTP-dependent Spine Enlargement Requires Synaptic Ca2+-permeable AMPARs Recruited by CaM-kinase I, J. Neurosci. Off. J. Soc. Neurosci 30 (2010) 11565–11575. 10.1523/JNEUROSCI.1746-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y, Structural and molecular remodeling of dendritic spine substructures during long-term potentiation, Neuron. 82 (2014) 444–459. 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mikhaylova M, Bär J, van Bommel B, Schätzle P, YuanXiang P, Raman R, Hradsky J, Konietzny A, Loktionov EY, Reddy PP, Lopez-Rojas J, Spilker C, Kobler O, Raza SA, Stork O, Hoogenraad CC, Kreutz MR, Caldendrin Directly Couples Postsynaptic Calcium Signals to Actin Remodeling in Dendritic Spines, Neuron. 97 (2018) 1110–1125.e14. 10.1016/j.neuron.2018.01.046. [DOI] [PubMed] [Google Scholar]
- [104].Tripathi A, Parikh ZS, Vora P, Frost EE, Pillai PP, pERK1/2 Peripheral Recruitment and Filopodia Protrusion Augment Oligodendrocyte Progenitor Cell Migration: Combined Effects of PDGF-A and Fibronectin, Cell. Mol. Neurobiol 37 (2017) 183–194. 10.1007/s10571-016-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kelley LC, Hayes KE, Ammer AG, Martin KH, Weed SA, Cortactin phosphorylated by ERK1/2 localizes to sites of dynamic actin regulation and is required for carcinoma lamellipodia persistence, PloS One. 5 (2010) e13847 10.1371/journal.pone.0013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Uruno T, Liu J, Zhang P, null Fan Yx C Egile, R. Li, S.C. Mueller, X. Zhan, Activation of Arp2/3 complex-mediated actin polymerization by cortactin, Nat. Cell Biol 3 (2001) 259–266. 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- [107].Lüscher C, Malenka RC, NMDA Receptor-Dependent Long-Term Potentiation and Long-Term Depression (LTP/LTD), Cold Spring Harb. Perspect. Biol 4 (2012). 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Redmond L, Kashani AH, Ghosh A, Calcium Regulation of Dendritic Growth via CaM Kinase IV and CREB-Mediated Transcription, Neuron. 34 (2002) 999–1010. 10.1016/S0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- [109].Pende M, Holtzclaw LA, Curtis JL, Russell JT, Gallo V, Glutamate regulates intracellular calcium and gene expression in oligodendrocyte progenitors through the activation of DL-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors., Proc. Natl. Acad. Sci 91 (1994) 3215–3219. 10.1073/pnas.91.8.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Kao S-C, Wu H, Xie J, Chang C-P, Ranish JA, Graef IA, Crabtree GR, Calcineurin/NFAT Signaling Is Required for Neuregulin-Regulated Schwann Cell Differentiation, Science. 323 (2009) 651–654. 10.1126/science.1166562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Weider M, Starost LJ, Groll K, Küspert M, Sock E, Wedel M, Fröb F, Schmitt C, Baroti T, Hartwig AC, Hillgärtner S, Piefke S, Fadler T, Ehrlich M, Ehlert C, Stehling M, Albrecht S, Jabali A, Schöler HR, Winkler J, Kuhlmann T, Wegner M, Nfat/calcineurin signaling promotes oligodendrocyte differentiation and myelination by transcription factor network tuning, Nat. Commun 9 (2018) 899 10.1038/s41467-018-03336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Macian F, NFAT proteins: key regulators of T-cell development and function, Nat. Rev. Immunol 5 (2005) 472 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- [113].Dietz L, Frommer F, Vogel A-L, Vaeth M, Serfling E, Waisman A, Buttmann M, Berberich-Siebelt F, NFAT1 deficit and NFAT2 deficit attenuate EAE via different mechanisms, Eur. J. Immunol 45 (2015) 1377–1389. 10.1002/eji.201444638. [DOI] [PubMed] [Google Scholar]
- [114].Fink CC, Meyer T, Molecular mechanisms of CaMKII activation in neuronal plasticity, Curr. Opin. Neurobiol 12 (2002) 293–299. 10.1016/S0959-4388(02)00327-6. [DOI] [PubMed] [Google Scholar]
- [115].Waggener CT, Dupree JL, Elgersma Y, Fuss B, CaMKII Regulates Oligodendrocyte Maturation and CNS Myelination, J. Neurosci 33 (2013) 10453–10458. 10.1523/JNEUROSCI.5875-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Carson MJ, Behringer RR, Brinster RL, McMorris FA, Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice, Neuron. 10 (1993) 729–740. [DOI] [PubMed] [Google Scholar]
- [117].Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB, Constitutively active Akt induces enhanced myelination in the CNS, J. Neurosci. Off. J. Soc. Neurosci 28 (2008) 7174–7183. 10.1523/JNEUROSCI.0150-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Narayanan SP, Flores AI, Wang F, Macklin WB, Akt signals through the mammalian target of rapamycin, mTOR, pathway to regulate central nervous system myelination, J. Neurosci. Off. J. Soc. Neurosci 29 (2009) 6860–6870. 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wahl SE, McLane LE, Bercury KK, Macklin WB, Wood TL, Mammalian Target of Rapamycin Promotes Oligodendrocyte Differentiation, Initiation and Extent of CNS Myelination, J. Neurosci 34 (2014) 4453–4465. 10.1523/JNEUROSCI.4311-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Bercury KK, Dai J, Sachs HH, Ahrendsen JT, Wood TL, Macklin WB, Conditional Ablation of Raptor or Rictor Has Differential Impact on Oligodendrocyte Differentiation and CNS Myelination, J. Neurosci 34 (2014) 4466–4480. 10.1523/JNEUROSCI.4314-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Xiao J, Ferner AH, Wong AW, Denham M, Kilpatrick TJ, Murray SS, Extracellular signal-regulated kinase 1/2 signaling promotes oligodendrocyte myelination in vitro: Erk1/2 promotes oligodendrocyte myelination, J. Neurochem 122 (2012) 1167–1180. 10.1111/j.1471-4159.2012.07871.x. [DOI] [PubMed] [Google Scholar]
- [122].Jeffries MA, Urbanek K, Torres L, Wendell SG, Rubio ME, Fyffe-Maricich SL, ERK1/2 Activation in Preexisting Oligodendrocytes of Adult Mice Drives New Myelin Synthesis and Enhanced CNS Function, J. Neurosci 36 (2016) 9186–9200. 10.1523/JNEUROSCI.1444-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ishii A, Furusho M, Dupree JL, Bansal R, Strength of ERK1/2 MAPK Activation Determines Its Effect on Myelin and Axonal Integrity in the Adult CNS, J. Neurosci 36 (2016) 6471–6487. 10.1523/JNEUROSCI.0299-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bruno MA, Cuello AC, Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade, Proc. Natl. Acad. Sci 103 (2006) 6735–6740. 10.1073/pnas.0510645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Cao P, Maximov A, Südhof TC, Activity-Dependent IGF-1 Exocytosis Is Controlled by the Ca2+-Sensor Synaptotagmin-10, Cell. 145 (2011) 300–311. 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sutton G, Chandler LJ, Activity-dependent NMDA receptor-mediated activation of protein kinase B/Akt in cortical neuronal cultures, J. Neurochem 82 (2002) 1097–1105. 10.1046/j.1471-4159.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- [127].Mameli M, Balland B, Luján R, Lüscher C, Rapid Synthesis and Synaptic Insertion of GluR2 for mGluR-LTD in the Ventral Tegmental Area, Science. 317 (2007) 530–533. 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- [128].Lundgaard I, Luzhynskaya A, Stockley JH, Wang Z, Evans KA, Swire M, Volbracht K, Gautier HOB, Franklin RJM, ffrench-Constant C, Attwell D, Káradóttir RT, Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes, PLOS Biol. 11 (2013) e1001743 10.1371/journal.pbio.1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Makinodan M, Rosen KM, Ito S, Corfas G, A Critical Period for Social Experience–Dependent Oligodendrocyte Maturation and Myelination, Science. 337 (2012) 1357–1360. 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, Woo PJ, Barres BA, Liddelow S, Vogel H, Monje M, Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment, Cell. 176 (2019) 43–55.e13. 10.1016/j.cell.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Geraghty AC, Gibson EM, Ghanem RA, Greene JJ, Ocampo A, Goldstein AK, Ni L, Yang T, Marton RM, Paşca SP, Greenberg ME, Longo FM, Monje M, Loss of Adaptive Myelination Contributes to Methotrexate Chemotherapy-Related Cognitive Impairment, Neuron. 0 (2019). 10.1016/j.neuron.2019.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Wong AW, Xiao J, Kemper D, Kilpatrick TJ, Murray SS, Oligodendroglial Expression of TrkB Independently Regulates Myelination and Progenitor Cell Proliferation, J. Neurosci 33 (2013) 4947–4957. 10.1523/JNEUROSCI.3990-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Nicholson M, Wood RJ, Fletcher JL, van den Buuse M, Murray SS, Xiao J, BDNF haploinsufficiency exerts a transient and regionally different influence upon oligodendroglial lineage cells during postnatal development, Mol. Cell. Neurosci 90 (2018) 12–21. 10.1016/j.mcn.2018.05.005. [DOI] [PubMed] [Google Scholar]
- [134].Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD, Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage, Nat. Neurosci 9 (2006) 173–179. 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Tsai H-H, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien A-C, Kuo CJ, Chan JR, Daneman R, Fancy SPJ, Oligodendrocyte precursors migrate along vasculature in the developing nervous system, Science. 351 (2016) 379–384. 10.1126/science.aad3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Choe Y, Huynh T, Pleasure SJ, Migration of Oligodendrocyte Progenitor Cells Is Controlled by Transforming Growth Factor β Family Proteins during Corticogenesis, J. Neurosci 34 (2014) 14973–14983. 10.1523/JNEUROSCI.1156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].de Castro F, Bribián A, Ortega MC, Regulation of oligodendrocyte precursor migration during development, in adulthood and in pathology, Cell. Mol. Life Sci 70 (2013) 4355–4368. 10.1007/s00018-013-1365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gautier HOB, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I, James F, Lao-Peregrin C, Reynolds R, Franklin RJM, Káradóttir RT, Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors, Nat. Commun 6 (2015) 8518 10.1038/ncomms9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Gudz TI, Komuro H, Macklin WB, Glutamate Stimulates Oligodendrocyte Progenitor Migration Mediated via an αv Integrin/Myelin Proteolipid Protein Complex, J. Neurosci 26 (2006) 2458–2466. 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Paez PM, Fulton DJ, Spreuer V, Handley V, Campagnoni CW, Macklin WB, Colwell C, Campagnoni AT, Golli myelin basic proteins regulate oligodendroglial progenitor cell migration through voltage-gated Ca++ influx, J. Neurosci. Off. J. Soc. Neurosci 29 (2009) 6663–6676. 10.1523/JNEUROSCI.5806-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Luyt K, Slade TP, Dorward JJ, Durant CF, Wu Y, Shigemoto R, Mundell SJ, Váradi A, Molnár E, Developing oligodendrocytes express functional GABAB receptors that stimulate cell proliferation and migration, J. Neurochem 100 (2007) 822–840. 10.1111/j.1471-4159.2006.04255.x. [DOI] [PubMed] [Google Scholar]
- [142].Xiao L, Hu C, Yang W, Guo D, Li C, Shen W, Liu X, Aijun H, Dan W, He C, NMDA receptor couples Rac1-GEF Tiam1 to direct oligodendrocyte precursor cell migration: NMDAR Couples Tiam1 for OPC Migration, Glia. 61 (2013) 2078–2099. 10.1002/glia.22578. [DOI] [PubMed] [Google Scholar]
- [143].Mangin J-M, Li P, Scafidi J, Gallo V, Experience-dependent regulation of NG2 progenitors in the developing barrel cortex, Nat. Neurosci 15 (2012) 1192–1194. 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Barres BA, Hart IK, Coles HSR, Burne JF, Voyvodic JT, Richardson WD, Raff MC, Cell death and control of cell survival in the oligodendrocyte lineage, Cell. 70 (1992) 31–46. 10.1016/0092-8674(92)90531-G. [DOI] [PubMed] [Google Scholar]
- [145].Trapp BD, Nishiyama A, Cheng D, Macklin W, Differentiation and Death of Premyelinating Oligodendrocytes in Developing Rodent Brain, J. Cell Biol 137 (1997) 459–468. 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kawai K, Itoh T, Itoh A, Horiuchi M, Wakayama K, Bannerman P, Garbern JY, Pleasure D, Lindsten T, Maintenance of the relative proportion of oligodendrocytes to axons even in the absence of BAX and BAK, Eur. J. Neurosci 30 (2009) 2030–2041. 10.1111/j.1460-9568.2009.06988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hill RA, Patel KD, Goncalves CM, Grutzendler J, Nishiyama A, Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division, Nat. Neurosci 17 (2014) 1518–1527. 10.1038/nn.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, Edgar JM, Yagensky O, Wichert SP, Agarwal A, Karram K, Renier N, Tessier-Lavigne M, Rossner MJ, Káradóttir RT, Nave K-A, A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination, Nat. Neurosci 20 (2017) 10–15. 10.1038/nn.4425. [DOI] [PubMed] [Google Scholar]
- [149].Leslie JR, Imai F, Fukuhara K, Takegahara N, Rizvi TA, Friedel RH, Wang F, Kumanogoh A, Yoshida Y, Ectopic myelinating oligodendrocytes in the dorsal spinal cord as a consequence of altered semaphorin 6D signaling inhibit synapse formation, Dev. Camb. Engl 138 (2011) 4085–4095. 10.1242/dev.066076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Meyer RC, Giddens MM, Schaefer SA, Hall RA, GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin, Proc. Natl. Acad. Sci 110 (2013) 9529–9534. 10.1073/pnas.1219004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Yang H-J, Vainshtein A, Maik-Rachline G, Peles E, G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination, Nat. Commun 7 (2016) 10884 10.1038/ncomms10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Morató X, Luján R, López-Cano M, Gandía J, Stagljar I, Watanabe M, Cunha RA, Fernández-Dueñas V, Ciruela F, The Parkinson’s disease-associated GPR37 receptor interacts with striatal adenosine A 2A receptor controlling its cell surface expression and function in vivo, Sci. Rep 7 (2017) 9452 10.1038/s41598-017-10147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Sun LO, Mulinyawe SB, Collins HY, Ibrahim A, Li Q, Simon DJ, Tessier-Lavigne M, Barres BA, Spatiotemporal Control of CNS Myelination by Oligodendrocyte Programmed Cell Death through the TFEB-PUMA Axis, Cell. 175 (2018) 1811–1826.e21. 10.1016/j.cell.2018.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Fancy SPJ, Baranzini SE, Zhao C, Yuk D-I, Irvine K-A, Kaing S, Sanai N, Franklin RJM, Rowitch DH, Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS, Genes Dev. 23 (2009) 1571–1585. 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A, Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB, Nat. Cell Biol 17 (2015) 288–299. 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Snaidero N, Möbius W, Czopka T, Hekking LHP, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, Nave K-A, Simons M, Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue, Cell. 156 (2014) 277–290. 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N, CNS live imaging reveals a new mechanism of myelination: the liquid croissant model, Glia. 59 (2011) 1841–1849. 10.1002/glia.21228. [DOI] [PubMed] [Google Scholar]
- [158].Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA, Individual Neuronal Subtypes Exhibit Diversity in CNS Myelination Mediated by Synaptic Vesicle Release, Curr. Biol 26 (2016) 1447–1455. 10.1016/j.cub.2016.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Redmond SA, Mei F, Eshed-Eisenbach Y, Osso LA, Leshkowitz D, Shen Y-AA, Kay JN, Aurrand-Lions M, Lyons DA, Peles E, Chan JR, Somatodendritic Expression of JAM2 Inhibits Oligodendrocyte Myelination, Neuron. 91 (2016) 824–836. 10.1016/j.neuron.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Nagappan-Chettiar S, Johnson-Venkatesh EM, Umemori H, Activity-dependent proteolytic cleavage of cell adhesion molecules regulates excitatory synaptic development and function, Neurosci. Res 116 (2017) 60–69. 10.1016/j.neures.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Elazar N, Vainshtein A, Golan N, Vijayaragavan B, Schaeren-Wiemers N, Eshed-Eisenbach Y, Peles E, Axoglial Adhesion by Cadm4 Regulates CNS Myelination, Neuron. 101 (2019) 224–231.e5. 10.1016/j.neuron.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hughes AN, Appel B, Oligodendrocytes express synaptic proteins that modulate myelin sheath formation, Nat. Commun 10 (2019) 1–15. 10.1038/s41467-019-12059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Choe H, Burtnick LD, Mejillano M, Yin HL, Robinson RC, Choe S, The calcium activation of gelsolin: insights from the 3A structure of the G4-G6/actin complex, J. Mol. Biol 324 (2002) 691–702. [DOI] [PubMed] [Google Scholar]
- [164].Wang Y, Shibasaki F, Mizuno K, Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin, J. Biol. Chem 280 (2005) 12683–12689. 10.1074/jbc.M411494200. [DOI] [PubMed] [Google Scholar]
- [165].Yergert KM, Hines JH, Appel B, Neuronal Activity Enhances mRNA Localization to Myelin Sheaths During Development, BioRxiv. (2019) 654616 10.1101/654616. [DOI] [Google Scholar]
- [166].Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P, Impaired adult myelination in the prefrontal cortex of socially isolated mice, Nat. Neurosci 15 (2012) 1621–1623. 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Koenning M, Jackson S, Hay CM, Faux C, Kilpatrick TJ, Willingham M, Emery B, Myelin Gene Regulatory Factor Is Required for Maintenance of Myelin and Mature Oligodendrocyte Identity in the Adult CNS, J. Neurosci 32 (2012) 12528–12542. 10.1523/JNEUROSCI.1069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Harris JJ, Attwell D, The Energetics of CNS White Matter, J. Neurosci 32 (2012) 356–371. 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Nave K-A, Myelination and support of axonal integrity by glia, Nature. 468 (2010) 244–252. 10.1038/nature09614. [DOI] [PubMed] [Google Scholar]
- [170].Tekkök SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR, Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity, J. Neurosci. Res 81 (2005) 644–652. 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- [171].Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B, In Vivo Evidence for Lactate as a Neuronal Energy Source, J. Neurosci 31 (2011) 7477–7485. 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave K-A, Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity, Nature. 485 (2012) 517–521. 10.1038/nature11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang P-W, Pellerin L, Magistretti PJ, Rothstein JD, Oligodendroglia metabolically support axons and contribute to neurodegeneration, Nature. 487 (2012) 443–448. 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Meyer N, Richter N, Fan Z, Siemonsmeier G, Pivneva T, Jordan P, Steinhäuser C, Semtner M, Nolte C, Kettenmann H, Oligodendrocytes in the Mouse Corpus Callosum Maintain Axonal Function by Delivery of Glucose, Cell Rep. 22 (2018) 2383–2394. 10.1016/j.celrep.2018.02.022. [DOI] [PubMed] [Google Scholar]
- [175].Saab AS, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Möbius W, Goetze B, Jahn HM, Huang W, Steffens H, Schomburg ED, Pérez-Samartín A, Pérez-Cerdá F, Bakhtiari D, Matute C, Löwel S, Griesinger C, Hirrlinger J, Kirchhoff F, Nave K-A, Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism, Neuron. 91 (2016) 119–132. 10.1016/j.neuron.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chuquet J, Quilichini P, Nimchinsky EA, Buzsáki G, Predominant enhancement of glucose uptake in astrocytes versus neurons during activation of the somatosensory cortex, J. Neurosci. Off. J. Soc. Neurosci 30 (2010) 15298–15303. 10.1523/JNEUROSCI.0762-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Orthmann-Murphy JL, Abrams CK, Scherer SS, Gap Junctions Couple Astrocytes and Oligodendrocytes, J. Mol. Neurosci. MN 35 (2008) 101–116. 10.1007/s12031-007-9027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Tress O, Maglione M, May D, Pivneva T, Richter N, Seyfarth J, Binder S, Zlomuzica A, Seifert G, Theis M, Dere E, Kettenmann H, Willecke K, Panglial Gap Junctional Communication is Essential for Maintenance of Myelin in the CNS, J. Neurosci 32 (2012) 7499–7518. 10.1523/JNEUROSCI.0392-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Etxeberria A, Mangin J-M, Aguirre A, Gallo V, Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum, Nat. Neurosci 13 (2010) 287–289. 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Sahel A, Ortiz FC, Kerninon C, Maldonado PP, Angulo MC, Nait-Oumesmar B, Alteration of synaptic connectivity of oligodendrocyte precursor cells following demyelination, Front. Cell. Neurosci 9 (2015). 10.3389/fncel.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Salinas PC, Wnt Signaling in the Vertebrate Central Nervous System: From Axon Guidance to Synaptic Function, Cold Spring Harb. Perspect. Biol 4 (2012) a008003–a008003. 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Ortiz FC, Habermacher C, Graciarena M, Houry P-Y, Nishiyama A, Oumesmar BN, Angulo MC, Neuronal activity in vivo enhances functional myelin repair, JCI Insight. 4 (2019). 10.1172/jci.insight.123434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Li Q, Houdayer T, Liu S, Belegu V, Induced Neural Activity Promotes an Oligodendroglia Regenerative Response in the Injured Spinal Cord and Improves Motor Function after Spinal Cord Injury, J. Neurotrauma 34 (2017) 3351–3361. 10.1089/neu.2016.4913. [DOI] [PubMed] [Google Scholar]
- [184].Chuderland D, Seger R, Calcium regulates ERK signaling by modulating its protein-protein interactions, Commun. Integr. Biol 1 (2008) 4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Wake H, Lee PR, Fields RD, Control of local protein synthesis and initial events in myelination by action potentials, Science. 333 (2011) 1647–1651. 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Caprariello AV, Batt CE, Zippe I, Romito-DiGiacomo RR, Karl M, Miller RH, Apoptosis of Oligodendrocytes during Early Development Delays Myelination and Impairs Subsequent Responses to Demyelination, J. Neurosci 35 (2015) 14031–14041. 10.1523/JNEUROSCI.1706-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


