Figure 4 |. Calcium signaling in mature oligodendrocytes.
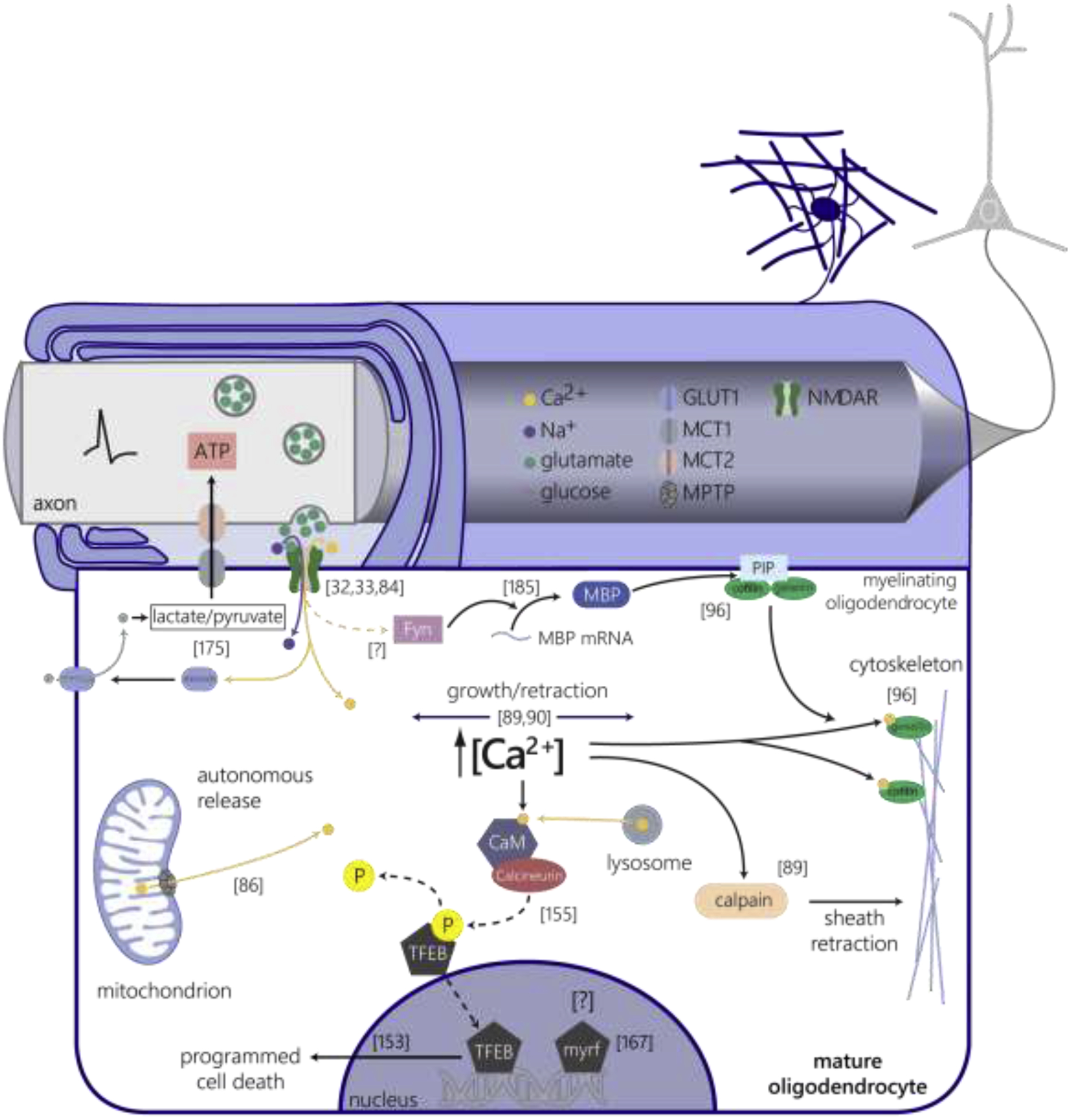
Dynamic bidirectional signaling between axons and mature oligodendrocytes regulates metabolic support, local protein translation, and gene expression. Mature oligodendrocytes sense and respond to neuronal glutamate release through NMDA receptors (green) expressed on the membrane in the periaxonal space [32,33,84]. A proportion of calcium elevations in mature myelin are derived from autonomous release through the mitochondrial permeability transition pore (MPTP, grey) in response to high mitochondrial respiration and production of ATP [86]. NMDA receptor-mediated calcium events increase the expression of the GLUT1 glucose transporter (light blue) on oligodendrocytes, which increases glucose (grey) entry and facilitates the delivery of lactate and pyruvate to active axons through monocarboxylate transporters 1 and 2 (grey, pink, [175]). Activity-dependent glutamatergic vesicle release increases the local translation of myelin basic protein (MBP) via a Fyn kinase-dependent mechanism [185], yet it is unknown whether changes in intracellular calcium modulate Fyn kinase activity to mediate this process. MBP binds to phosphatidylinositol 4,5-bisphosphate (PIP2) to release the calcium-dependent cytoskeletal reorganizing proteins gelsolin and cofilin from the membrane to induce actin depolymerization during myelin wrapping [96]. Neuronal activity-dependent elevations in intra-sheath calcium concentration regulate sheath growth and retraction in zebrafish development [89,90]. Oligodendrocyte-specific inhibition of calpain proteases increases the number of myelin sheaths produced, implicating calcium-induced proteolysis in sheath retraction [89]. Lysosomal calcium signaling activates calcineurin, which dephosphorylates the transcription factor EB (TFEB) and induces nuclear translocation to regulate autophagy [155]. TFEB regulates mature oligodendrocyte integration via activation of apoptotic pathways [153], yet it is unknown whether this process is regulated by intracellular calcium-calcineurin signaling. Mature oligodendrocyte-specific genetic deletion of the membrane-associated transcription factor myelin regulatory factor (myrf) results in oligodendrocyte degeneration [167], yet whether activity-dependent axonal signals regulate gene expression to support myelin maintenance or sheath remodeling is unknown.
